In the ionic compounds LiF, NaCl, KBr, and RbI, the measured cation–anion distances are 2.01 Å (Li–F), 2.82 Å (Na–Cl), 3.30 Å (K–Br), and 3.67 Å (Rb–I), respectively. c. What estimates of the cation–anion distance would you obtain for these four compounds using neutral atom bonding atomic radii? Are these estimates as accurate as the estimates using ionic radii?
Ch.7 - Periodic Properties of the Elements

Brown15th EditionChemistry: The Central ScienceISBN: 9780137542970Not the one you use?Change textbook
Chapter 7, Problem 40
Which of the ions Ni2+, Fe2+, Co3+, and Pt2+ has an electron configuration of 𝑛𝑑6(𝑛=3,4,5,…)?
a. Ni2+
b. Fe2+
c. Co3+
d. Pt2+
e. More than one of these
 Verified step by step guidance
Verified step by step guidance1
insert step 1> Determine the electron configuration of the neutral atoms: Ni, Fe, Co, and Pt. For transition metals, the general order is to fill the 4s orbital before the 3d orbital.
insert step 2> For each ion, remove electrons starting from the outermost shell, which is typically the s orbital, followed by the d orbital if necessary.
insert step 3> Calculate the electron configuration for each ion: Ni^{2+}, Fe^{2+}, Co^{3+}, and Pt^{2+}.
insert step 4> Identify which ion has an electron configuration ending in nd^6.
insert step 5> Compare the electron configurations to determine if more than one ion has the nd^6 configuration.

Verified video answer for a similar problem:
This video solution was recommended by our tutors as helpful for the problem above.
Video duration:
6mWas this helpful?
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Electron Configuration
Electron configuration describes the distribution of electrons in an atom's orbitals. It is represented using a notation that indicates the energy levels and sublevels occupied by electrons. For transition metals, the d-orbitals play a significant role, and understanding how to write and interpret these configurations is essential for predicting chemical behavior and properties.
Recommended video:
Guided course

Electron Configuration Example
Oxidation States
Oxidation states indicate the degree of oxidation of an atom in a compound, reflecting the number of electrons lost or gained. Transition metals can exhibit multiple oxidation states, which affects their electron configurations. Knowing the oxidation state of an ion helps in determining its electron configuration, especially for ions like Ni2+, Fe2+, Co3+, and Pt2+.
Recommended video:
Guided course

Oxidation Numbers
d-Orbital Filling
The d-orbitals are filled according to specific rules, including the Aufbau principle, Hund's rule, and the Pauli exclusion principle. For transition metals, the filling of d-orbitals is crucial for understanding their chemical properties and electron configurations. The notation 𝑛𝑑6 indicates that there are six electrons in the d-orbitals, which is key to identifying the correct ion from the given options.
Recommended video:
Guided course
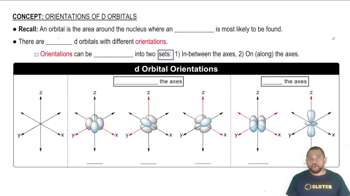
d Orbital Orientations
Related Practice
Textbook Question
Textbook Question
Write the electron configurations for the following ions, and determine which have noble-gas configurations.
a. Ru3+
b. As3−
c. Y3+
d. Pd2+
e. Pb2+
f. Au3+
Textbook Question
a. Write an equation for the second electron affinity of chlorine.
b. Would you predict a positive or a negative quantity for this process?
Textbook Question
rue or false: If the electron affinity for an element is a negative number, then the anion of the element is more stable than the neutral atom.
Textbook Question
Write equations that show the processes that describe the first, second, and third ionization energies of an aluminum atom. Which process would require the least amount of energy?
