In the ionic compounds LiF, NaCl, KBr, and RbI, the measured cation–anion distances are 2.01 Å (Li–F), 2.82 Å (Na–Cl), 3.30 Å (K–Br), and 3.67 Å (Rb–I), respectively. b. Calculate the difference between the experimentally measured ion–ion distances and the ones predicted from Figure 7.8.

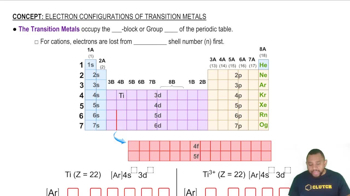
Give three examples of +2 ions that have an electron configuration of nd10 (n = 3, 4, 5...).
 Verified step by step guidance
Verified step by step guidanceKey Concepts
Electron Configuration
Transition Metals
Ionic Charge and Stability
In the ionic compounds LiF, NaCl, KBr, and RbI, the measured cation–anion distances are 2.01 Å (Li–F), 2.82 Å (Na–Cl), 3.30 Å (K–Br), and 3.67 Å (Rb–I), respectively. c. What estimates of the cation–anion distance would you obtain for these four compounds using neutral atom bonding atomic radii? Are these estimates as accurate as the estimates using ionic radii?
Write the electron configurations for the following ions, and determine which have noble-gas configurations.
a. Ru3+
b. As3−
c. Y3+
d. Pd2+
e. Pb2+
f. Au3+
Which of the ions Ni2+, Fe2+, Co3+, and Pt2+ has an electron configuration of 𝑛𝑑6(𝑛=3,4,5,…)?
a. Ni2+
b. Fe2+
c. Co3+
d. Pt2+
e. More than one of these
a. Write an equation for the second electron affinity of chlorine.
b. Would you predict a positive or a negative quantity for this process?
rue or false: If the electron affinity for an element is a negative number, then the anion of the element is more stable than the neutral atom.
