Consider S, Cl, and K and their most common ions. (a) List the atoms in order of increasing size.

True or false? c. S2− is larger than K+.
 Verified step by step guidance
Verified step by step guidance
Verified video answer for a similar problem:
Key Concepts
Ionic Radius
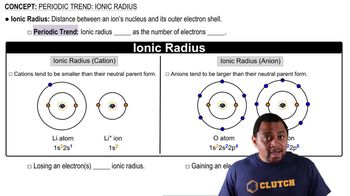
Charge and Size Relationship

Comparison of Ions
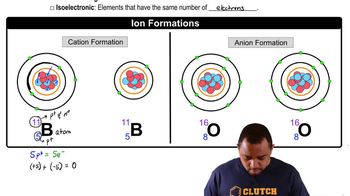
Consider S, Cl, and K and their most common ions. (b) List the ions in order of increasing size. (c) Explain any differences in the orders of the atomic and ionic sizes.
Arrange each of the following sets of atoms and ions in order of increasing size. Se2−,Te2−, Se
In the ionic compounds LiF, NaCl, KBr, and RbI, the measured cation–anion distances are 2.01 Å (Li–F), 2.82 Å (Na–Cl), 3.30 Å (K–Br), and 3.67 Å (Rb–I), respectively. b. Calculate the difference between the experimentally measured ion–ion distances and the ones predicted from Figure 7.8.
In the ionic compounds LiF, NaCl, KBr, and RbI, the measured cation–anion distances are 2.01 Å (Li–F), 2.82 Å (Na–Cl), 3.30 Å (K–Br), and 3.67 Å (Rb–I), respectively. c. What estimates of the cation–anion distance would you obtain for these four compounds using neutral atom bonding atomic radii? Are these estimates as accurate as the estimates using ionic radii?
Write the electron configurations for the following ions, and determine which have noble-gas configurations.
a. Ru3+
b. As3−
c. Y3+
d. Pd2+
e. Pb2+
f. Au3+
