Write a balanced equation for the reaction that occurs in each of the following cases: (b) Strontium oxide is added to water. (c) A fresh surface of lithium metal is exposed to oxygen gas. (d) Sodium metal reacts with molten sulfur.
Ch.7 - Periodic Properties of the Elements

Brown15th EditionChemistry: The Central ScienceISBN: 9780137542970Not the one you use?Change textbook
Chapter 7, Problem 71
(a) As described in Section 7.7, the alkali metals react with hydrogen to form hydrides and react with halogens to form halides. Compare the roles of hydrogen and halogens in these reactions. Write balanced equations for the reaction of fluorine with calcium and for the reaction of hydrogen with calcium. (b) What is the oxidation number and electron configuration of calcium in each product?
 Verified step by step guidance
Verified step by step guidance1
Identify the products in the reaction involving calcium. If the reaction or products are not specified, consider common compounds of calcium such as calcium oxide (CaO), calcium chloride (CaCl2), and calcium carbonate (CaCO3).
Determine the oxidation number of calcium in each product. Remember that the oxidation number of calcium is typically +2 in its compounds, as it tends to lose two electrons to achieve a stable electron configuration.
Write the electron configuration for calcium in its elemental state. The atomic number of calcium is 20, so its electron configuration is \$1s^2 2s^2 2p^6 3s^2 3p^6 4s^2$.
Understand that upon ionization when forming compounds, calcium loses the two electrons in its outermost 4s orbital. This results in the electron configuration for Ca^2+ as \$1s^2 2s^2 2p^6 3s^2 3p^6$.
Apply this understanding to each product identified in step 1, confirming that the electron configuration of calcium in its ionic form (Ca^2+) remains consistent across different compounds.

Verified video answer for a similar problem:
This video solution was recommended by our tutors as helpful for the problem above.
Video duration:
10mWas this helpful?
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Oxidation Number
The oxidation number, or oxidation state, is a value that represents the total number of electrons an atom either gains or loses when it forms a compound. It helps in understanding the electron transfer in redox reactions. For calcium, which is an alkaline earth metal, the oxidation number is typically +2 in compounds, indicating it loses two electrons.
Recommended video:
Guided course
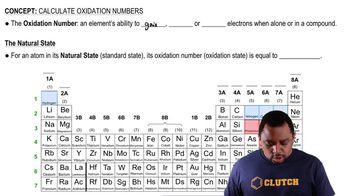
Oxidation Numbers
Electron Configuration
Electron configuration describes the distribution of electrons in an atom's orbitals. For calcium, the electron configuration is 1s² 2s² 2p⁶ 3s², indicating that it has two electrons in its outermost shell. This configuration is crucial for determining how calcium interacts chemically, particularly in forming compounds.
Recommended video:
Guided course
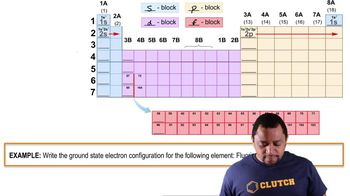
Electron Configuration Example
Chemical Products
In a chemical reaction, products are the substances formed as a result of the reaction. The oxidation state and electron configuration of an element can change depending on the products formed. Understanding the nature of these products is essential for determining the final oxidation state and electron configuration of calcium in the context of the reaction.
Recommended video:
Guided course

Production of Hydrogen Example
Related Practice
Textbook Question
1
views
Textbook Question
Write a balanced equation for the reaction that occurs in each of the following cases: (a) Cesium is added to water.
1
views
Textbook Question
Write a balanced equation for the reaction that occurs in each of the following cases: (c) Sodium reacts with oxygen.
1
views
Textbook Question
Potassium and hydrogen react to form the ionic compound potassium hydride. (b) Use data in Figures 7.10 and 7.12 to determine the energy change in kJ/mol for the following two reactions:
K(g) + H(g) → K+(g) + H-(g)
K(g) + H(g) → K-(g) + H+(g)
Textbook Question
Little is known about the properties of astatine, At, because of its rarity and high radioactivity. Nevertheless, it is possible for us to make many predictions about its properties. (a) Do you expect the element to be a gas, liquid, or solid at room temperature?
