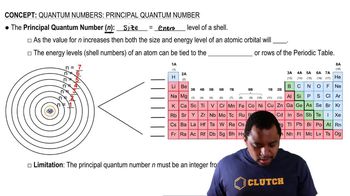
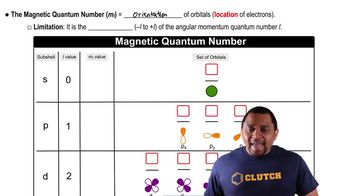
Textbook Question
Calculate the uncertainty in the position of (a) an electron moving at a speed of 13.00 ± 0.012 × 105 m/s (b) a neutron moving at this same speed. (The masses of an electron and a neutron are given in the table of fundamental constants in the inside cover of the text.)