Textbook Question
(a) For n = 4, what are the possible values of l?

 Verified step by step guidance
Verified step by step guidance
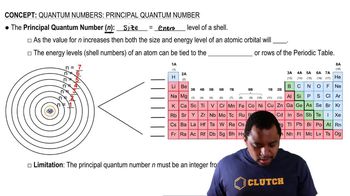

(a) For n = 4, what are the possible values of l?
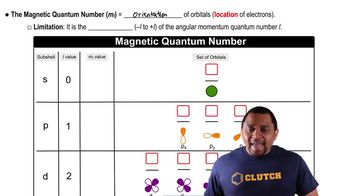
How many unique combinations of the quantum numbers l and 𝑚𝑙 are there when b. n = 4?
Give the numerical values of n and l corresponding to each of the following orbital designations: (a) 3p (b) 2s (c) 4f
Give the values for n, l, and 𝑚𝑙 for a. each orbital in the 2p subshell
A certain orbital of the hydrogen atom has n = 4 and l = 2. a. What are the possible values of ml for this orbital?
A certain orbital of the hydrogen atom has n = 4 and l = 2. b. What are the possible values of ms for the orbital?