Sketch the shape and orientation of the following types of orbitals: (a) px, (b) dz2, (c) dx2 - y2.

(b) If we add one electron to form the He atom, would your answer to part (a) change?
 Verified step by step guidance
Verified step by step guidance
Verified video answer for a similar problem:
Key Concepts
Atomic Structure
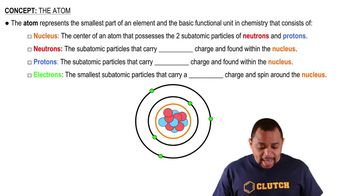
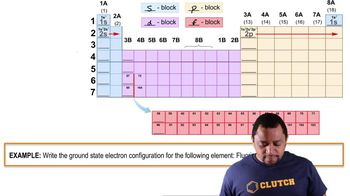
Ionization and Electron Configuration
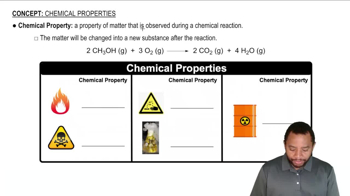
Chemical Properties of Elements
(a) With reference to Figure 6.19, what is the relationship between the number of nodes in an s orbital and the value of the principal quantum number?
(a) For an He+ ion, do the 2s and 2p orbitals have the same energy? If not, which orbital has a lower energy?
(a) The average distance from the nucleus of a 3s electron in a chlorine atom is smaller than that for a 3p electron. In light of this fact, which orbital is higher in energy?
Two possible electron configurations for an Li atom are shown here. (c) In the absence of an external magnetic field, can we say that one electron configuration has a lower energy than the other? If so, which one has the lowest energy?
An experiment called the Stern–Gerlach experiment helped establish the existence of electron spin. In this experiment, a beam of silver atoms is passed through a magnetic field, which deflects half of the silver atoms in one direction and half in the opposite direction. The separation between the two beams increases as the strength of the magnetic field increases. (a) What is the electron configuration for a silver atom?
