For the table that follows, write which orbital goes with the quantum numbers. Don't worry about x, y, z subscripts. If the quantum numbers are not allowed, write 'not allowed.' n l ml Orbital 2 1 -1 2p (example) 1 0 0 3 -3 2 3 2 -2 2 0 -1 0 0 0 4 2 1 5 3 0
Ch.6 - Electronic Structure of Atoms

Brown15th EditionChemistry: The Central ScienceISBN: 9780137542970Not the one you use?Change textbook
Chapter 6, Problem 66a
(a) With reference to Figure 6.19, what is the relationship between the number of nodes in an s orbital and the value of the principal quantum number?

 Verified step by step guidance
Verified step by step guidance1
Examine the provided graphs for the 1s, 2s, and 3s orbitals, noting the number of nodes in each.
Identify that the 1s orbital has 0 nodes, the 2s orbital has 1 node, and the 3s orbital has 2 nodes.
Recall that the principal quantum number (n) represents the energy level of the orbital.
Understand that the number of nodes in an s orbital is given by the formula: number of nodes = n - 1.
Conclude that as the principal quantum number (n) increases, the number of nodes in the s orbital also increases according to the relationship: number of nodes = n - 1.

Verified video answer for a similar problem:
This video solution was recommended by our tutors as helpful for the problem above.
Video duration:
1mWas this helpful?
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
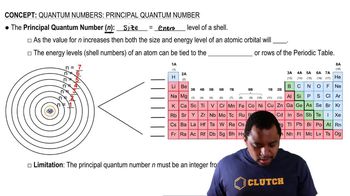
Principal Quantum Number (n)
The principal quantum number (n) indicates the energy level of an electron in an atom and is a positive integer (1, 2, 3, ...). As n increases, the electron's average distance from the nucleus increases, leading to higher energy levels. Each value of n corresponds to a different electron shell, with higher n values allowing for more complex orbital shapes and sizes.
Recommended video:
Guided course
Principal Quantum Number
Nodes in Orbitals
Nodes are regions in an atomic orbital where the probability of finding an electron is zero. The number of nodes in an orbital is directly related to the principal quantum number; specifically, the number of nodes increases as n increases. For s orbitals, the number of nodes is given by the formula n-1, meaning that a 1s orbital has no nodes, a 2s has one node, and a 3s has two nodes.
Recommended video:
Guided course

Radial Nodes Example
s Orbitals
s orbitals are spherical in shape and are characterized by their principal quantum number. Each s orbital can hold a maximum of two electrons and has a specific number of nodes based on its n value. The graphical representation of s orbitals shows how the probability of finding an electron varies with distance from the nucleus, highlighting the presence of nodes as the principal quantum number increases.
Recommended video:
Guided course
Molecular Orbital Theory
Related Practice
Textbook Question
1
views
Textbook Question
Sketch the shape and orientation of the following types of orbitals: (a) s, (b) pz, (c) dxy.
Textbook Question
Sketch the shape and orientation of the following types of orbitals: (a) px, (b) dz2, (c) dx2 - y2.
1
views
Textbook Question
(a) For an He+ ion, do the 2s and 2p orbitals have the same energy? If not, which orbital has a lower energy?
Textbook Question
(b) If we add one electron to form the He atom, would your answer to part (a) change?
Textbook Question
(a) The average distance from the nucleus of a 3s electron in a chlorine atom is smaller than that for a 3p electron. In light of this fact, which orbital is higher in energy?
