Methanol (CH3OH) is used as a fuel in race cars. (c) Calculate the heat produced by combustion per liter of methanol. Methanol has a density of 0.791 g/mL.
Ch.5 - Thermochemistry

Brown15th EditionChemistry: The Central ScienceISBN: 9780137542970Not the one you use?Change textbook
Chapter 5, Problem 88a
Use bond enthalpies in Table 5.4 to estimate H for each of the following reactions: (a)
 Verified step by step guidance
Verified step by step guidance1
Identify the bonds broken and formed in the reaction. Breaking bonds requires energy (endothermic), while forming bonds releases energy (exothermic).
Use the bond enthalpy values from Table 5.4 to calculate the total energy required to break all the bonds in the reactants.
Calculate the total energy released by forming all the bonds in the products using the bond enthalpy values.
Subtract the total energy released (from forming bonds) from the total energy required (for breaking bonds) to find the overall change in enthalpy (ΔH) for the reaction.
Remember that a negative ΔH indicates an exothermic reaction, while a positive ΔH indicates an endothermic reaction.

Verified video answer for a similar problem:
This video solution was recommended by our tutors as helpful for the problem above.
Video duration:
8mWas this helpful?
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Bond Enthalpy
Bond enthalpy, or bond dissociation energy, is the amount of energy required to break a bond in a molecule in the gas phase. It is typically expressed in kilojoules per mole (kJ/mol) and varies depending on the type of bond and the surrounding atoms. Understanding bond enthalpy is crucial for estimating the energy changes in chemical reactions, as it allows for the calculation of the total energy required to break bonds in reactants and the energy released when new bonds are formed in products.
Recommended video:
Guided course

Enthalpy of Formation
Hess's Law
Hess's Law states that the total enthalpy change for a reaction is the same, regardless of the number of steps taken to achieve the reaction. This principle allows chemists to calculate the enthalpy change of a reaction by summing the enthalpy changes of individual steps, which can be particularly useful when direct measurement is difficult. In the context of using bond enthalpies, Hess's Law enables the estimation of the overall reaction enthalpy by considering the bonds broken and formed.
Recommended video:
Guided course
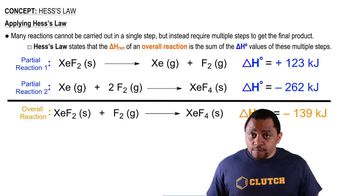
Hess's Law
Reaction Enthalpy (ΔH)
The reaction enthalpy, denoted as ΔH, represents the change in enthalpy during a chemical reaction. It can be calculated using the formula ΔH = Σ(bond enthalpies of bonds broken) - Σ(bond enthalpies of bonds formed). A positive ΔH indicates an endothermic reaction (energy absorbed), while a negative ΔH indicates an exothermic reaction (energy released). Understanding how to calculate ΔH using bond enthalpies is essential for predicting the energy dynamics of chemical reactions.
Recommended video:
Guided course

Enthalpy of Formation
Related Practice
Textbook Question
Textbook Question
Methanol (CH3OH) is used as a fuel in race cars. (d) Calculate the mass of CO2 produced per kJ of heat emitted.
Textbook Question
Ethane, C2H6, is an alkane with one C─C bond and six C─H bonds (Section 2.9). a. Use enthalpies of formation given in Appendix C to calculate Δ𝐻 for the reaction C2H6(𝑔)→2C(𝑔)+6H(𝑔). b. Use the result from part (a) and the value of 𝐷(C─H) from Table 5.4 to estimate the bond enthalpy 𝐷(C─C). c. How large is the difference between the value calculated for in part (b) and the value given in Table 5.4?
Textbook Question
Consider the reaction 2 H2(g) + O2(g) → 2 H2O(l). (a) Use the bond enthalpies in Table 5.4 to estimate H for this reaction, ignoring the fact that water is in the liquid state.
