A 2.200-g sample of quinone (C6H4O2) is burned in a bomb calorimeter whose total heat capacity is 7.854 kJ/°C. The temperature of the calorimeter increases from 23.44 to 30.57 °C. b. What is the heat of combustion per mole of quinone?

Under constant-volume conditions, the heat of combustion of benzoic acid (C6H5COOH) is 26.38 kJ/g. A 2.760-g sample of benzoic acid is burned in a bomb calorimeter. The temperature of the calorimeter increases from 21.60 to 29.93 °C. c. Suppose that in changing samples, a portion of the water in the calorimeter were lost. In what way, if any, would this change the heat capacity of the calorimeter?
 Verified step by step guidance
Verified step by step guidance
Verified video answer for a similar problem:
Key Concepts
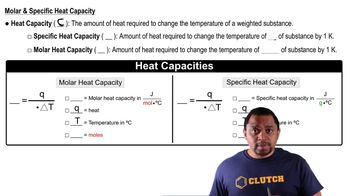
Heat Capacity
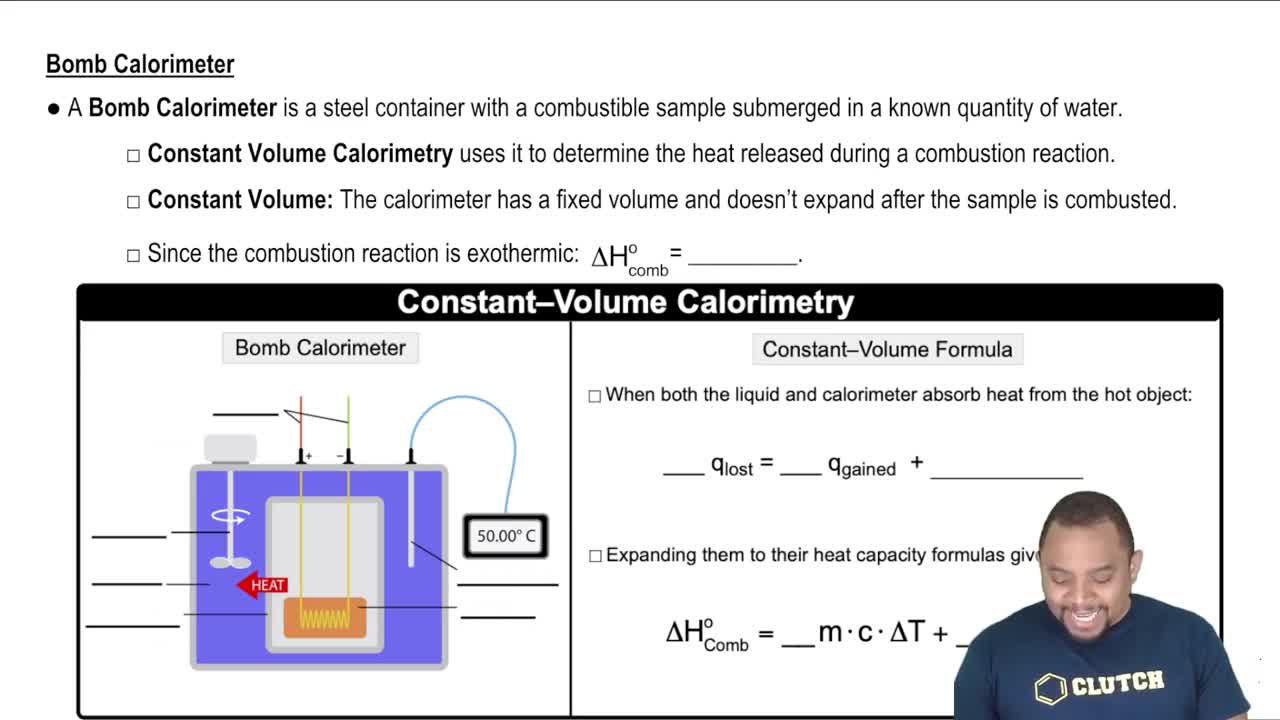
Bomb Calorimeter
Specific Heat Capacity

A 1.800-g sample of phenol (C6H5OH) was burned in a bomb calorimeter whose total heat capacity is 11.66 kJ/°C. The temperature of the calorimeter plus contents increased from 21.36 to 26.37 °C. a. Write a balanced chemical equation for the bomb calorimeter reaction.
A 1.800-g sample of phenol (C6H5OH) was burned in a bomb calorimeter whose total heat capacity is 11.66 kJ/°C. The temperature of the calorimeter plus contents increased from 21.36 to 26.37 °C. b. What is the heat of combustion per gram of phenol?
Consider the following hypothetical reactions: A → B ΔH = +30 kJ B → C ΔH = +60 kJ (b) Construct an enthalpy diagram for substances A, B, and C, and show how Hess's law applies.
Calculate the enthalpy change for the reaction P4O6(s) + 2 O2(g) → P4O10(s) given the following enthalpies of reaction: P4(s) + 3 O2(g) → P4O6(s) ΔH = -1640.1 kJ P4(s) + 5 O2(g) → P4O10(s) ΔH = -2940.1 kJ
From the enthalpies of reaction 2 C(s) + O2(g) → 2 CO(g) ΔH = -221.0 kJ 2 C(s) + O2(g) + 4 H2(g) → 2 CH3OH(g) ΔH = -402.4 kJ Calculate ΔH for the reaction CO(g) + 2 H2(g) → CH3OH(g)
