Under constant-volume conditions, the heat of combustion of benzoic acid (C6H5COOH) is 26.38 kJ/g. A 2.760-g sample of benzoic acid is burned in a bomb calorimeter. The temperature of the calorimeter increases from 21.60 to 29.93 °C. c. Suppose that in changing samples, a portion of the water in the calorimeter were lost. In what way, if any, would this change the heat capacity of the calorimeter?

From the enthalpies of reaction 2 C(s) + O2(g) → 2 CO(g) ΔH = -221.0 kJ 2 C(s) + O2(g) + 4 H2(g) → 2 CH3OH(g) ΔH = -402.4 kJ Calculate ΔH for the reaction CO(g) + 2 H2(g) → CH3OH(g)
 Verified step by step guidance
Verified step by step guidance
Verified video answer for a similar problem:
Key Concepts
Enthalpy of Reaction

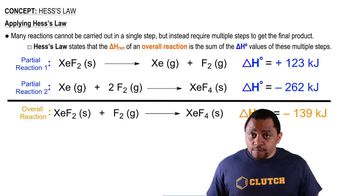
Hess's Law
Stoichiometry in Reactions

Consider the following hypothetical reactions: A → B ΔH = +30 kJ B → C ΔH = +60 kJ (b) Construct an enthalpy diagram for substances A, B, and C, and show how Hess's law applies.
Calculate the enthalpy change for the reaction P4O6(s) + 2 O2(g) → P4O10(s) given the following enthalpies of reaction: P4(s) + 3 O2(g) → P4O6(s) ΔH = -1640.1 kJ P4(s) + 5 O2(g) → P4O10(s) ΔH = -2940.1 kJ
The concentration of alcohol 1CH3CH2OH2 in blood, called the 'blood alcohol concentration' or BAC, is given in units of grams of alcohol per 100 mL of blood. The legal definition of intoxication, in many states of the United States, is that the BAC is 0.08 or higher. What is the concentration of alcohol, in terms of molarity, in blood if the BAC is 0.08?
Given the data N2(g) + O2(g) → 2 NO(g) ΔH = +180.7 kJ 2 NO(g) + O2(g) → 2 NO2(g) ΔH = -113.1 kJ 2 N2O(g) → 2 N2(g) + O2(g) ΔH = -163.2 kJ use Hess's law to calculate ΔH for the reaction N2O(g) + NO2(g) → 3 NO(g)
We can use Hess's law to calculate enthalpy changes that cannot be measured. One such reaction is the conversion of methane to ethane: 2 CH4(g) → C2H6(g) + H2(g) Calculate the ΔH° for this reaction using the following thermochemical data: CH4(g) + 2 O2(g) → CO2(g) + 2 H2O(l) ΔH° = -890.3 kJ 2 H2(g) + O2(g) → 2 H2O(l) H° = -571.6 kJ 2 C2H6(g) + 7 O2(g) → 4 CO2(g) + 6 H2O(l) ΔH° = -3120.8 kJ
