Ethane, C2H6, is an alkane with one C─C bond and six C─H bonds (Section 2.9). a. Use enthalpies of formation given in Appendix C to calculate Δ𝐻 for the reaction C2H6(𝑔)→2C(𝑔)+6H(𝑔). b. Use the result from part (a) and the value of 𝐷(C─H) from Table 5.4 to estimate the bond enthalpy 𝐷(C─C). c. How large is the difference between the value calculated for in part (b) and the value given in Table 5.4?
Ch.5 - Thermochemistry

Brown15th EditionChemistry: The Central ScienceISBN: 9780137542970Not the one you use?Change textbook
Chapter 5, Problem 90
Consider the reaction H2(g) + Br2(l) → 2 HBr(g). (b) Without doing a calculation, predict whether your estimate in part (a) is more negative or less negative than the true reaction enthalpy.
 Verified step by step guidance
Verified step by step guidance1
Step 1: Understand the reaction: The reaction given is H2(g) + Br2(l) → 2 HBr(g). This is a synthesis reaction where hydrogen gas reacts with liquid bromine to form hydrogen bromide gas.
Step 2: Consider the states of matter: Note that H2 is a gas, Br2 is a liquid, and HBr is a gas. The phase change from liquid to gas for Br2 will require energy input, which affects the enthalpy.
Step 3: Analyze bond energies: Breaking bonds requires energy, while forming bonds releases energy. In this reaction, the H-H and Br-Br bonds are broken, and new H-Br bonds are formed.
Step 4: Compare bond energies: Typically, the energy required to break the H-H and Br-Br bonds is less than the energy released when forming two H-Br bonds, suggesting an exothermic reaction.
Step 5: Predict the enthalpy change: Since the reaction is likely exothermic, the true reaction enthalpy is negative. If your estimate in part (a) did not fully account for the energy released in forming H-Br bonds, it might be less negative than the true reaction enthalpy.
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Enthalpy of Reaction
The enthalpy of reaction, or reaction enthalpy, is the heat change that occurs during a chemical reaction at constant pressure. It is a key concept in thermodynamics, indicating whether a reaction is exothermic (releases heat) or endothermic (absorbs heat). Understanding this helps predict the energy changes associated with the formation of products from reactants.
Recommended video:
Guided course

Enthalpy of Formation
Standard Enthalpy of Formation
The standard enthalpy of formation is the change in enthalpy when one mole of a compound is formed from its elements in their standard states. This concept is crucial for estimating reaction enthalpies, as it allows for the calculation of the overall energy change by using tabulated values for the reactants and products involved in the reaction.
Recommended video:
Guided course

Enthalpy of Formation
Hess's Law
Hess's Law states that the total enthalpy change for a reaction is the same, regardless of the number of steps taken to complete the reaction. This principle allows chemists to calculate the enthalpy change of a reaction indirectly by summing the enthalpy changes of individual steps, which is particularly useful when direct measurement is difficult or impossible.
Recommended video:
Guided course
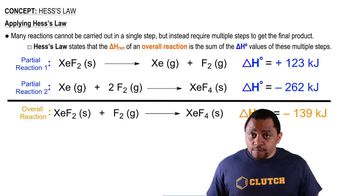
Hess's Law
Related Practice
Textbook Question
Textbook Question
Use bond enthalpies in Table 5.4 to estimate H for each of the following reactions: (a)
Textbook Question
Consider the reaction 2 H2(g) + O2(g) → 2 H2O(l). (a) Use the bond enthalpies in Table 5.4 to estimate H for this reaction, ignoring the fact that water is in the liquid state.
Textbook Question
(b) A particular chip snack food is composed of 12% protein, 14% fat, and the rest carbohydrate. What percentage of the calorie content of this food is fat?
1
views
Textbook Question
(a) A serving of a particular ready-to-serve chicken noodle soup contains 2.5 g fat, 14 g carbohydrate, and 7 g protein. Estimate the number of Calories in a serving.
1
views
