Consider the combustion of isopropanol, C3H7OH(l), which is the primary component of rubbing alcohol: C3H7OH(l) + 9/2 O2(g) → 3 CO2(g) + 4 H2O(l) ΔH = -2248 kJ a. What is the enthalpy change for the reverse reaction?

Consider the decomposition of liquid benzene, C6H6(l), to gaseous acetylene, C2H2(g): C6H6(l) → 3 C2H2(g) ΔH = +630 kJ (b) What is H for the formation of 1 mol of acetylene?
 Verified step by step guidance
Verified step by step guidance
Verified video answer for a similar problem:
Key Concepts
Thermochemistry
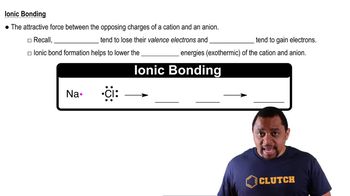
Enthalpy of Formation

Stoichiometry

Consider the combustion of isopropanol, C3H7OH(l), which is the primary component of rubbing alcohol: C3H7OH(l) + 9/2 O2(g) → 3 CO2(g) + 4 H2O(l) ΔH = -2248 kJ (b) Balance the forward reaction with whole-number coefficients. What is ΔH for the reaction represented by this equation?
Consider the decomposition of liquid benzene, C6H6(l), to gaseous acetylene, C2H2(g): C6H6(l) → 3 C2H2(g) ΔH = +630 kJ (a) What is the enthalpy change for the reverse reaction?
Consider the decomposition of liquid benzene, C6H6(l), to gaseous acetylene, C2H2(g): C6H6(l) → 3 C2H2(g) ΔH = +630 kJ (c) Which is more likely to be thermodynamically favored, the forward reaction or the reverse reaction?
Consider the decomposition of liquid benzene, C6H6(l), to gaseous acetylene, C2H2(g): C6H6(l) → 3 C2H2(g) ΔH = +630 kJ (d) If C6H6(g) were consumed instead of C6H6(l), would you expect the magnitude of ΔH to increase, decrease, or stay the same? Explain.
b. What are the units of specific heat?
