Imagine a container placed in a tub of water, as depicted in the accompanying diagram. (a) If the contents of the container are the system and heat is able to flow through the container walls, what qualitative changes will occur in the temperatures of the system and in its surroundings? From the system's perspective, is the process exothermic or endothermic?
Ch.5 - Thermochemistry

Brown15th EditionChemistry: The Central ScienceISBN: 9780137542970Not the one you use?Change textbook
Chapter 5, Problem 11
Consider the combustion of a single molecule of CH4(g), forming H2O(l) as a product. (b) A typical X-ray light source has an energy of 8 keV (see inside back cover for conversion between eV and J). Is the energy released by the combustion of a CH4 molecule larger or smaller than the energy of an X-ray from this source?
 Verified step by step guidance
Verified step by step guidance1
Step 1: Write the balanced chemical equation for the combustion of methane (CH_4). The reaction is: CH_4(g) + 2O_2(g) -> CO_2(g) + 2H_2O(l).
Step 2: Determine the energy released by the combustion of one mole of CH_4. This is typically given as the standard enthalpy change of combustion (ΔH_comb) for methane, which can be found in thermodynamic tables.
Step 3: Convert the energy released per mole of CH_4 to energy released per molecule. Use Avogadro's number (6.022 x 10^23 molecules/mol) for this conversion.
Step 4: Convert the energy of the X-ray light source from keV to joules. Use the conversion factor: 1 eV = 1.602 x 10^-19 J.
Step 5: Compare the energy released per molecule of CH_4 with the energy of the X-ray. Determine if the energy released by the combustion is larger or smaller than the energy of the X-ray.
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Combustion Reaction
Combustion is a chemical reaction that typically involves the reaction of a hydrocarbon with oxygen to produce carbon dioxide and water, releasing energy in the form of heat and light. In the case of methane (CH4), the reaction can be represented as CH4 + 2O2 → CO2 + 2H2O. Understanding the stoichiometry and energy changes associated with this reaction is crucial for comparing the energy released to other forms of energy, such as X-ray photons.
Recommended video:
Guided course

Combustion Apparatus
Energy Units and Conversion
Energy can be expressed in various units, including electronvolts (eV) and joules (J). In this context, 1 eV is equivalent to approximately 1.6 x 10^-19 J. The question involves comparing the energy released from the combustion of methane to the energy of X-ray photons, which are typically measured in keV (kiloelectronvolts). Understanding how to convert between these units is essential for making accurate comparisons.
Recommended video:
Guided course

Conversion Factors
Energy of X-ray Photons
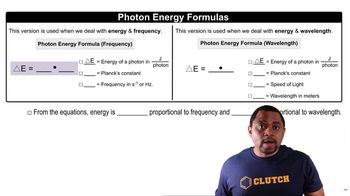
X-ray photons are high-energy electromagnetic radiation, and their energy can be calculated using the formula E = hf, where E is energy, h is Planck's constant, and f is the frequency of the radiation. For an X-ray source with an energy of 8 keV, this corresponds to a specific energy value in joules. Knowing the energy of X-ray photons allows for a direct comparison with the energy released during the combustion of methane.
Recommended video:
Guided course
Photon Energy Formulas
Related Practice
Textbook Question
1
views
Textbook Question
In the accompanying cylinder diagram, a chemical process occurs at constant temperature and pressure. (a) Is the sign of w indicated by this change positive or negative?
2
views
Textbook Question
Consider the two diagrams that follow. (d) Would similar relationships hold for the work involved in each process?
2
views
Textbook Question
(b) Why does increasing the temperature cause a solid substance to change in succession from a solid to a liquid to a gas?
2
views
Textbook Question
(a) What is the electrostatic potential energy (in joules) between two electrons that are separated by 62 pm?
