Textbook Question
Imagine that you are climbing a mountain. Which of the following are state functions? a. The distance you walk during your climb to the top
1
views

 Verified step by step guidance
Verified step by step guidance

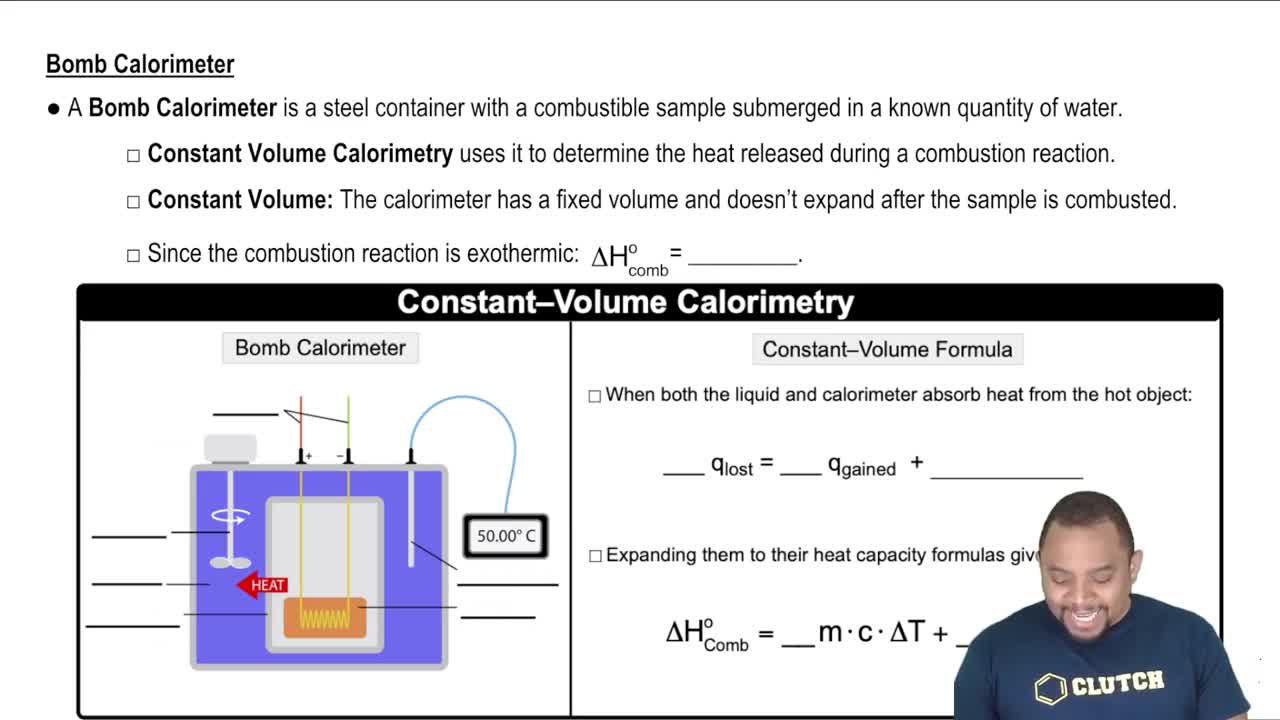
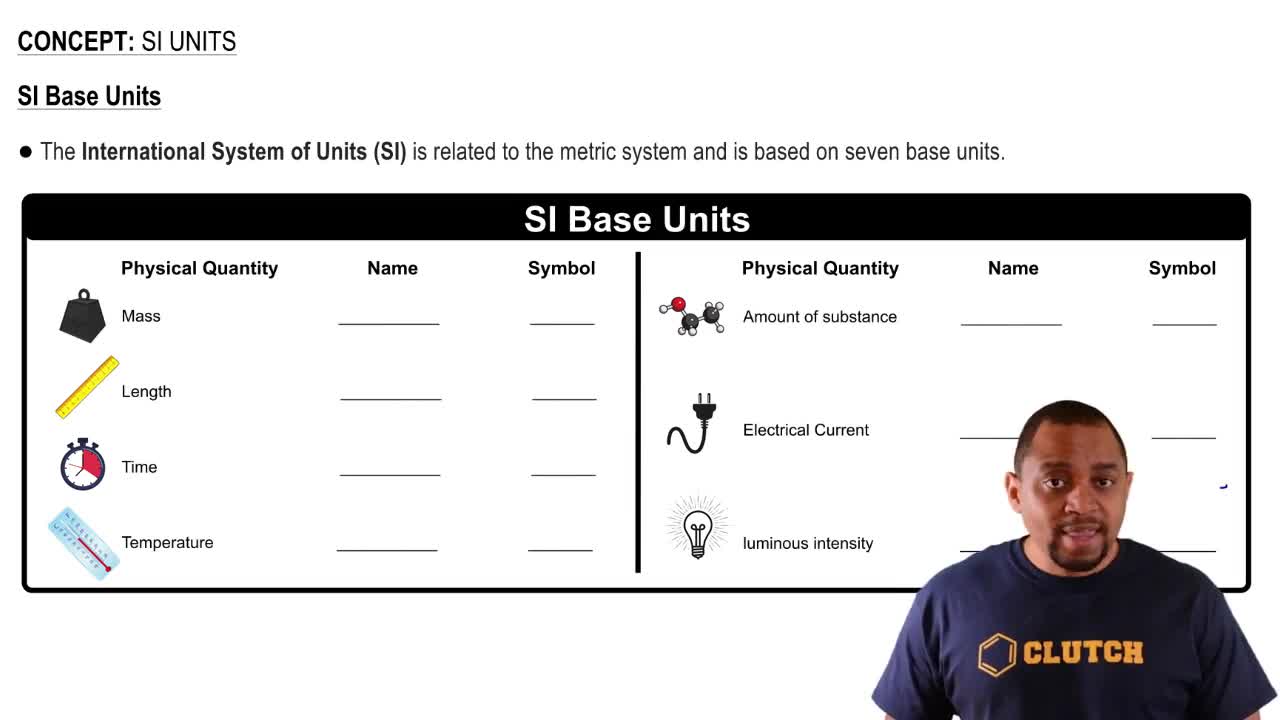
Imagine that you are climbing a mountain. Which of the following are state functions? a. The distance you walk during your climb to the top
Imagine that you are climbing a mountain. Which of the following are state functions? b. The change in elevation during the climb
During a normal breath, our lungs expand about 0.50 L against an external pressure of 1.0 atm. How much work is involved in this process (in J)?