Separate samples of a solution of an unknown ionic compound are treated with dilute AgNO3, Pb1NO322, and BaCl2. Precipitates form in all three cases. Which of the following could be the anion of the unknown salt: Br-, CO32-, NO3-?

Which of the following solutions is the most acidic? a. 0.2 M LiOH b. 0.2 M HI c. 1.0 M methanol (CH3OH)
 Verified step by step guidance
Verified step by step guidance
Verified video answer for a similar problem:
Key Concepts
Acidity and pH
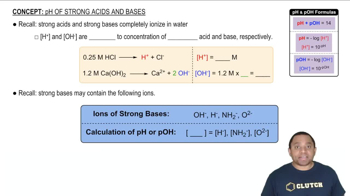
Strong vs. Weak Acids
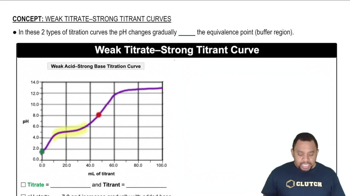
Neutralization and Basicity

You know that an unlabeled bottle contains an aqueous solution of one of the following: AgNO3,CaCl2, or Al2(SO4)3. You take a portion of the solution and add an aqueous solution of Ba(NO3)2 to it, and observe that a white solid precipitates Then you take another portion of the unlabeled solution and add an aqueous solution of NaCl to it; nothing appears to happen. What is the most likely identity of the solution in the unlabeled bottle: silver nitrate, calcium chloride, aluminum sulfate?
Three solutions are mixed together to form a single solution; in the final solution, there are 0.2 mol Pb1CH3COO)2, 0.1 mol Na2S, and 0.1 mol CaCl2 present. What solid(s) will precipitate?
Which of the following solutions is the most basic? a. 0.6𝑀 NH3 b. 0.150 M KOH c. 0.100𝑀Ba(OH)2
State whether each of the following statements is true or false. Justify your answer in each case. (a) Sulfuric acid is a monoprotic acid.
State whether each of the following statements is true or false. Justify your answer in each case. (b) HCl is a weak acid.
