Which of the following solutions is the most basic? a. 0.6𝑀 NH3 b. 0.150 M KOH c. 0.100𝑀Ba(OH)2

State whether each of the following statements is true or false. Justify your answer in each case. (c) Methanol is a base.
 Verified step by step guidance
Verified step by step guidance
Verified video answer for a similar problem:
Key Concepts
Acid-Base Theory

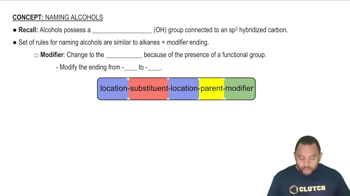
Properties of Alcohols
Solvent Effects on Acid-Base Behavior

State whether each of the following statements is true or false. Justify your answer in each case. (a) Sulfuric acid is a monoprotic acid.
State whether each of the following statements is true or false. Justify your answer in each case. (b) HCl is a weak acid.
State whether each of the following statements is true or false. Justify your answer in each case. (a) NH3 contains no OH- ions, and yet its aqueous solutions are basic
State whether each of the following statements is true or false. Justify your answer in each case. (b) HF is a strong acid.
State whether each of the following statements is true or false. Justify your answer in each case.
(c) Although sulfuric acid is a strong electrolyte, an aqueous solution of H2SO4 contains more HSO4- ions than SO42- ions.
