Textbook Question
Give the empirical formula of each of the following compounds if a sample contains a.0.0130 mol C, 0.0390 mol H, and 0.0065 mol O

 Verified step by step guidance
Verified step by step guidance
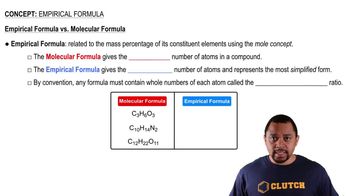

Give the empirical formula of each of the following compounds if a sample contains a.0.0130 mol C, 0.0390 mol H, and 0.0065 mol O
Give the empirical formula of each of the following compounds if a sample contains b.11.66 g iron and 5.01 g oxygen
Give the empirical formula of each of the following compounds if a sample contains c.40.0% C, 6.7% H, and 53.3% O by mass.
Determine the empirical formula of each of the following compounds if a sample contains b. 5.28 g Sn and 3.37 g F;
Determine the empirical formula of each of the following compounds if a sample contains c. 87.5% N and 12.5% H by mass.
Determine the empirical formulas of the compounds with the following compositions by mass: a. 10.4% C, 27.8% S, and 61.7% Cl