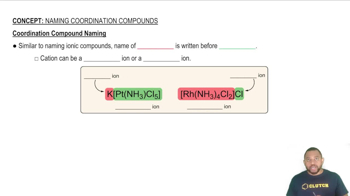
Sketch the structure of the complex in each of the following compounds and give the full compound name:
a. cis-[Co(NH3)4(H2O)2] (NO3)2

 Verified step by step guidance
Verified step by step guidance

Sketch the structure of the complex in each of the following compounds and give the full compound name:
a. cis-[Co(NH3)4(H2O)2] (NO3)2
Sketch the structure of the complex in each of the following compounds and give the full compound name:
b. Na2[Ru(H2O)Cl5]
Sketch the structure of the complex in each of the following compounds and give the full compound name:
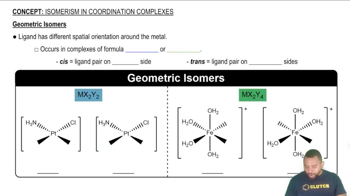
d. cis-[Ru(en)2Cl2]
The molecule dimethylphosphinoethane [(CH3)2PCH2CH2P(CH3)2, which is abbreviated dmpe] is used as a ligand for some complexes that serve as catalysts. A complex that contains this ligand is Mo(CO)4(dmpe) .
a. Draw the Lewis structure for dmpe, and compare it with ethylenediamine as a coordinating ligand.
b. What is the oxidation state of Mo in Na2[Mo(CN)2(CO)2(dmpe)] ?
c. Sketch the structure of the [Mo(CN)2(CO)2(dmpe)]2- ion, including all the possible isomers.