Textbook Question
Sketch the structure of the complex in each of the following compounds and give the full compound name:
a. cis-[Co(NH3)4(H2O)2] (NO3)2

 Verified step by step guidance
Verified step by step guidance
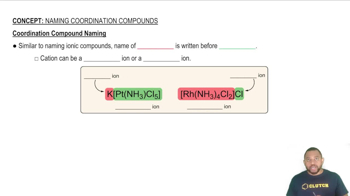


Sketch the structure of the complex in each of the following compounds and give the full compound name:
a. cis-[Co(NH3)4(H2O)2] (NO3)2
Sketch the structure of the complex in each of the following compounds and give the full compound name:
c. trans-NH3[Co(C2O4)2(H2O)2]
Sketch the structure of the complex in each of the following compounds and give the full compound name:
d. cis-[Ru(en)2Cl2]