Ch.23 - Transition Metals and Coordination Chemistry

Brown15th EditionChemistry: The Central ScienceISBN: 9780137542970Not the one you use?Change textbook
Chapter 23, Problem 36
Complete the exercises below. Write the formula for each of the following compounds, being sure to use brackets to indicate the coordination sphere: e.g., tris(ethylenediamine)rhodium(III), tris(oxalato)cobaltate(III).
 Verified step by step guidance
Verified step by step guidance1
Identify the central metal atom or ion in the coordination compound. This is typically a transition metal and is often indicated in the name of the compound.
Determine the oxidation state of the central metal. This is usually given in Roman numerals in the name of the compound, such as (III) for a +3 oxidation state.
Identify the ligands attached to the central metal. Ligands are molecules or ions that donate electron pairs to the metal. In the examples given, ethylenediamine and oxalato are the ligands.
Determine the number of each type of ligand. Prefixes like 'tris' indicate the number of ligands, with 'tris' meaning three.
Write the chemical formula by placing the central metal and its oxidation state outside the brackets, and the ligands inside the brackets, using the appropriate prefixes to indicate the number of each ligand.
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Coordination Compounds
Coordination compounds consist of a central metal atom or ion bonded to surrounding molecules or ions, known as ligands. The arrangement of these ligands around the metal center forms a coordination sphere, which is crucial for determining the compound's properties and reactivity. Understanding the nature of the ligands and their bonding is essential for writing correct formulas.
Recommended video:
Guided course
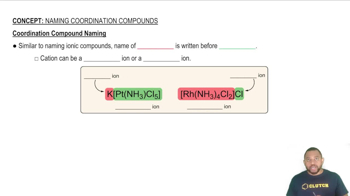
Coordination Compound Naming
Ligands
Ligands are ions or molecules that can donate a pair of electrons to a metal center in a coordination compound. They can be classified as monodentate (binding through one atom) or polydentate (binding through multiple atoms). The type and number of ligands influence the geometry and stability of the coordination complex, which is important when writing the compound's formula.
Recommended video:
Guided course

Ligands Example
Nomenclature of Coordination Compounds
The nomenclature of coordination compounds follows specific rules set by the International Union of Pure and Applied Chemistry (IUPAC). This includes naming the ligands first, followed by the metal center, and indicating the oxidation state of the metal in Roman numerals. Brackets are used to denote the coordination sphere, which helps clarify the structure of the compound in its formula.
Recommended video:
Guided course

Coordination Compound Naming
Related Practice
