Ch.23 - Transition Metals and Coordination Chemistry

Brown15th EditionChemistry: The Central ScienceISBN: 9780137542970Not the one you use?Change textbook
Chapter 23, Problem 39
Complete the exercises below. Consider the following three complexes: (Complex 1) [Co(NH₃)₄Br₂]Cl (Complex 2) [Pd(NH₃)₂(ONO)₂] (Complex 3) [V(en)₂Cl₂]⁺. Which of the three complexes can have: a. geometric isomers, b. linkage isomers, c. optical isomers, d. coordination-sphere isomers?
 Verified step by step guidance
Verified step by step guidance1
Step 1: Identify the coordination number and geometry of each complex. For example, determine if the complex is octahedral, square planar, or tetrahedral based on the number of ligands and the metal center.
Step 2: Analyze each complex for geometric isomerism. Geometric isomers occur in complexes with specific geometries, such as octahedral or square planar, where ligands can occupy different positions relative to each other.
Step 3: Examine each complex for linkage isomerism. Linkage isomers arise when a ligand can bind to the metal center through different atoms. Check if any ligands in the complexes have multiple donor atoms.
Step 4: Investigate each complex for optical isomerism. Optical isomers are non-superimposable mirror images, which can occur in chiral complexes, often with bidentate ligands creating an asymmetrical arrangement.
Step 5: Consider coordination-sphere isomerism for each complex. Coordination-sphere isomers differ in the ligands directly attached to the metal versus those outside the coordination sphere, such as counterions.
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Isomerism in Coordination Compounds
Isomerism refers to the phenomenon where compounds with the same molecular formula exhibit different structural or spatial arrangements. In coordination chemistry, isomers can be classified into several types, including geometric, linkage, optical, and coordination-sphere isomers, each defined by specific structural characteristics and bonding arrangements.
Recommended video:
Guided course
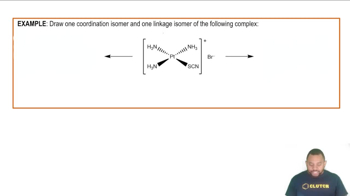
Isomerism in Coordination Complexes Example
Geometric Isomers
Geometric isomers arise from the different spatial arrangements of ligands around a central metal atom in a coordination complex. For example, in octahedral complexes, ligands can be arranged in cis or trans configurations, leading to distinct isomers with different physical and chemical properties.
Recommended video:
Guided course
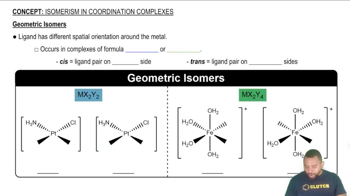
Geometric Isomers
Linkage Isomers
Linkage isomers occur when a ligand can coordinate to the metal center through different atoms. For instance, in complexes with ambidentate ligands, such as thiocyanate (SCN⁻), the ligand can bind through sulfur or nitrogen, resulting in different isomeric forms that can exhibit varied reactivity and properties.
Recommended video:
Guided course
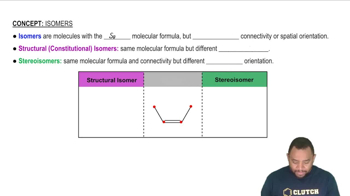
Isomers
Related Practice
