Ch.23 - Transition Metals and Coordination Chemistry

Brown15th EditionChemistry: The Central ScienceISBN: 9780137542970Not the one you use?Change textbook
Chapter 23, Problem 41
Complete the exercises below. A four-coordinate complex MA₂B₂ is prepared and found to have two different isomers. Is it possible to determine from this information whether the complex is square planar or tetrahedral? If so, which is it?
 Verified step by step guidance
Verified step by step guidance1
Step 1: Understand the coordination geometry options for a four-coordinate complex. A four-coordinate complex can either be square planar or tetrahedral.
Step 2: Consider the possible isomers for each geometry. In a square planar complex, the ligands can be arranged in a cis or trans configuration, leading to two different isomers. In a tetrahedral complex, all positions are equivalent, so it typically does not have geometric isomers.
Step 3: Analyze the given information. The problem states that the complex MA₂B₂ has two different isomers.
Step 4: Determine which geometry allows for two isomers. Since a tetrahedral complex does not typically have geometric isomers, the presence of two isomers suggests a square planar geometry.
Step 5: Conclude that the complex is square planar, as this geometry allows for the existence of two different isomers (cis and trans).
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Coordination Geometry
Coordination geometry refers to the spatial arrangement of ligands around a central metal atom in a complex. In four-coordinate complexes, the two common geometries are tetrahedral and square planar. The geometry influences the properties and reactivity of the complex, making it essential to understand when analyzing isomerism.
Recommended video:
Guided course
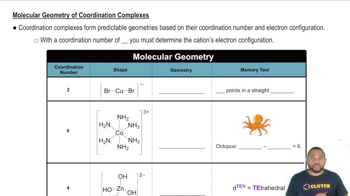
Molecular Geometry of Coordination Complexes
Isomerism in Coordination Compounds
Isomerism in coordination compounds occurs when two or more compounds have the same formula but different arrangements of atoms. For MA₂B₂ complexes, the presence of two isomers suggests that the ligands can be arranged in distinct ways, which can indicate the geometry of the complex. Understanding the types of isomers, such as geometric and optical isomers, is crucial for determining the structure.
Recommended video:
Guided course
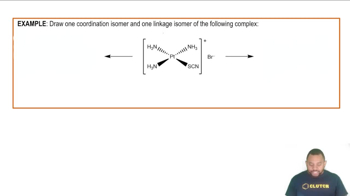
Isomerism in Coordination Complexes Example
Square Planar vs. Tetrahedral Complexes
Square planar complexes typically exhibit cis and trans isomerism due to the arrangement of ligands in a two-dimensional plane, while tetrahedral complexes do not show this type of isomerism. The presence of two isomers in the MA₂B₂ complex suggests a square planar geometry, as tetrahedral complexes would not yield different isomers under these conditions.
Recommended video:
Guided course
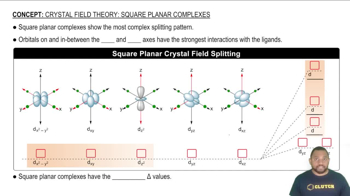
Square planar complexes show the most complex splitting pattern.
Related Practice
