Ch.22 - Chemistry of the Nonmetals

Brown15th EditionChemistry: The Central ScienceISBN: 9780137542970Not the one you use?Change textbook
Chapter 22, Problem 48
Complete the exercises below. Write a balanced equation for each of the following reactions. a. Hydrogen selenide can be prepared by the reaction of an aqueous acid solution on aluminum selenide. b. Sodium thiosulfate is used to remove excess Cl₂ from chlorine-bleached fabrics. The thiosulfate ion forms SO₄²⁻ and elemental sulfur, while Cl₂ is reduced to Cl⁻.
 Verified step by step guidance
Verified step by step guidance1
Identify the reactants and products for each reaction. For reaction (a), the reactants are hydrogen selenide (H₂Se) and aluminum selenide (Al₂Se₃) with an aqueous acid. For reaction (b), the reactants are sodium thiosulfate (Na₂S₂O₃) and chlorine (Cl₂).
Write the unbalanced chemical equations for each reaction. For reaction (a), the unbalanced equation is: Al₂Se₃ + H⁺ → H₂Se + Al³⁺. For reaction (b), the unbalanced equation is: Na₂S₂O₃ + Cl₂ → SO₄²⁻ + S + Cl⁻.
Balance the atoms for each element in the equations. Start with the most complex molecule or the element that appears in the least number of compounds. For reaction (a), balance the selenium and aluminum atoms first. For reaction (b), balance the sulfur and chlorine atoms first.
Ensure that the charge is balanced in each equation. For reaction (a), check that the charges on both sides of the equation are equal. For reaction (b), ensure that the total charge on the reactants side equals the total charge on the products side.
Verify that the number of atoms for each element and the total charge are balanced in the final equations. Adjust coefficients as necessary to achieve a balanced equation for both reactions.
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Balancing Chemical Equations
Balancing chemical equations is essential to ensure that the law of conservation of mass is upheld, meaning the number of atoms of each element must be the same on both sides of the equation. This involves adjusting coefficients in front of compounds to achieve equal numbers of each type of atom. For example, in the reaction of aluminum selenide with an acid, one must account for all reactants and products to write a balanced equation.
Recommended video:
Guided course

Balancing Chemical Equations
Acid-Base Reactions
Acid-base reactions involve the transfer of protons (H⁺ ions) between reactants. In the context of the first reaction, an aqueous acid reacts with aluminum selenide, leading to the formation of hydrogen selenide. Understanding the properties of acids and bases, including their ability to donate or accept protons, is crucial for predicting the products of such reactions.
Recommended video:
Guided course

Acid-Base Reaction
Redox Reactions
Redox (reduction-oxidation) reactions involve the transfer of electrons between species, resulting in changes in oxidation states. In the second reaction, sodium thiosulfate acts as a reducing agent, reducing chlorine gas (Cl₂) to chloride ions (Cl⁻) while itself being oxidized to sulfate ions (SO₄²⁻) and elemental sulfur. Recognizing the roles of oxidizing and reducing agents is vital for understanding the electron transfer processes in these reactions.
Recommended video:
Guided course
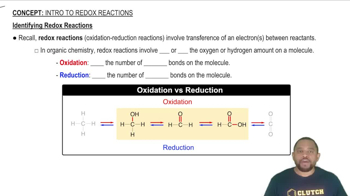
Identifying Redox Reactions
Related Practice
