Ch.22 - Chemistry of the Nonmetals

Brown15th EditionChemistry: The Central ScienceISBN: 9780137542970Not the one you use?Change textbook
Chapter 22, Problem 26
Complete the exercises below. Identify the following hydrides as ionic, metallic, or molecular: a. B₂H₆, b. RbH, c. Th₄H₁.₅.
 Verified step by step guidance
Verified step by step guidance1
Step 1: Understand the types of hydrides. Ionic hydrides are formed between hydrogen and highly electropositive metals, typically alkali or alkaline earth metals. Metallic hydrides involve transition metals and have metallic bonding. Molecular hydrides are covalent compounds formed with nonmetals or metalloids.
Step 2: Analyze compound a, B₂H₆ (diborane). Boron is a metalloid and forms covalent bonds with hydrogen, indicating that B₂H₆ is a molecular hydride.
Step 3: Analyze compound b, RbH. Rubidium (Rb) is an alkali metal, which is highly electropositive. When combined with hydrogen, it forms an ionic hydride.
Step 4: Analyze compound c, Th₄H₁.₅. Thorium (Th) is an actinide and a transition metal. Hydrides of transition metals are typically metallic hydrides.
Step 5: Summarize the findings: a. B₂H₆ is a molecular hydride, b. RbH is an ionic hydride, c. Th₄H₁.₅ is a metallic hydride.
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Types of Chemical Bonds
Chemical bonds can be classified into three main types: ionic, metallic, and covalent (molecular). Ionic bonds form between metals and nonmetals through the transfer of electrons, resulting in charged ions. Metallic bonds occur between metal atoms, characterized by a 'sea of electrons' that allows for conductivity and malleability. Covalent bonds, or molecular bonds, involve the sharing of electrons between nonmetals.
Recommended video:
Guided course
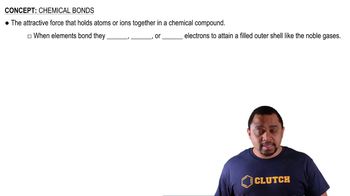
Chemical Bonds
Hydrides
Hydrides are compounds formed between hydrogen and other elements. They can be classified based on the type of bonding present. Ionic hydrides, like RbH, consist of hydrogen anions and metal cations, while molecular hydrides, such as B₂H₆, involve covalent bonding between hydrogen and nonmetals. Understanding the nature of the elements involved helps in determining the type of hydride.
Recommended video:
Guided course
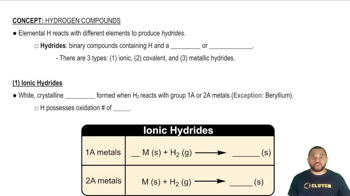
Ionic Hydrides
Periodic Trends and Element Properties
The properties of elements, including their tendency to form ionic, metallic, or molecular compounds, are influenced by their position in the periodic table. Metals, typically found on the left side, tend to lose electrons and form ionic bonds, while nonmetals, located on the right, often gain electrons or share them, leading to covalent bonds. Transition metals can exhibit metallic bonding due to their unique electron configurations.
Recommended video:
Guided course
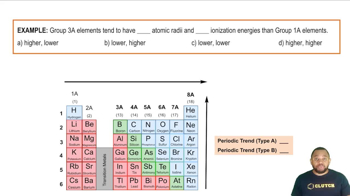
Main Group Elements: Periodic Trends Example
Related Practice
