Ch.22 - Chemistry of the Nonmetals

Brown15th EditionChemistry: The Central ScienceISBN: 9780137542970Not the one you use?Change textbook
Chapter 22, Problem 25
Complete the exercises below. Identify the following hydrides as ionic, metallic, or molecular: a. BaH₂, b. H₂Te, c. TiH₁.₇.
 Verified step by step guidance
Verified step by step guidance1
Step 1: Understand the types of hydrides. Hydrides can be classified into three main types: ionic hydrides, metallic hydrides, and molecular hydrides. Ionic hydrides typically form between hydrogen and alkali or alkaline earth metals. Metallic hydrides involve transition metals, and molecular hydrides are usually formed with nonmetals.
Step 2: Analyze BaH₂. Barium (Ba) is an alkaline earth metal. Hydrides formed with alkaline earth metals are typically ionic. Therefore, BaH₂ is likely an ionic hydride.
Step 3: Analyze H₂Te. Tellurium (Te) is a nonmetal. Hydrides formed with nonmetals are usually molecular. Therefore, H₂Te is likely a molecular hydride.
Step 4: Analyze TiH₁.₇. Titanium (Ti) is a transition metal. Hydrides formed with transition metals are typically metallic. Therefore, TiH₁.₇ is likely a metallic hydride.
Step 5: Summarize the classifications. Based on the analysis, BaH₂ is an ionic hydride, H₂Te is a molecular hydride, and TiH₁.₇ is a metallic hydride.
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Ionic Compounds
Ionic compounds are formed when atoms transfer electrons, resulting in the formation of charged ions. These compounds typically consist of a metal and a non-metal, where the metal donates electrons and the non-metal accepts them. Ionic bonds are characterized by strong electrostatic forces between the oppositely charged ions, leading to high melting and boiling points.
Recommended video:
Guided course

Ionic Compounds Naming
Molecular Compounds
Molecular compounds are formed when two or more non-metals share electrons through covalent bonds. These compounds consist of discrete molecules, and their properties differ significantly from ionic compounds. Molecular compounds generally have lower melting and boiling points and can exist in various states (solid, liquid, gas) at room temperature, depending on the strength of intermolecular forces.
Recommended video:
Guided course
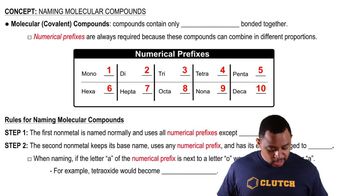
Naming Molecular Compounds
Metallic Compounds
Metallic compounds consist of metal atoms that share a 'sea of electrons' allowing for conductivity and malleability. In metallic bonding, electrons are not bound to any specific atom, which enables the metal atoms to slide past each other without breaking the bond. This unique structure gives metals their characteristic properties, such as luster and the ability to conduct electricity and heat.
Recommended video:
Guided course
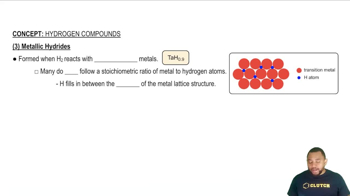
Metallic Hydrides
Related Practice
