Despite the similarities in the chemical reactivity of elements in the lanthanide series, their abundances in Earth's crust vary by two orders of magnitude. This graph shows the relative abundance as a function of atomic number. Which of the following statements best explains the sawtooth variation across the series? (a) The elements with an odd atomic number lie above the belt of stability. (b) The elements with an odd atomic number lie below the belt of stability. (c) The elements with an even atomic number have a magic number of protons. (d) Pairs of protons have a special stability.
Ch.21 - Nuclear Chemistry

Brown15th EditionChemistry: The Central ScienceISBN: 9780137542970Not the one you use?Change textbook
Chapter 21, Problem 27
Which statement best explains why nuclear transmutations involving neutrons are generally easier to accomplish than those involving protons or alpha particles? (a) Neutrons are not a magic number particle. (b) Neutrons do not have an electrical charge. (c) Neutrons are smaller than protons or alpha particles. (d) Neutrons are attracted to the nucleus even at long distances, whereas protons and alpha particles are repelled.
 Verified step by step guidance
Verified step by step guidance1
Step 1: Understand the concept of nuclear transmutation, which involves changing one element into another by altering the number of protons in the nucleus.
Step 2: Recognize that neutrons, protons, and alpha particles are all subatomic particles that can be used to induce nuclear transmutations.
Step 3: Consider the role of electrical charge in nuclear reactions. Neutrons are neutral, protons are positively charged, and alpha particles (which consist of 2 protons and 2 neutrons) are also positively charged.
Step 4: Analyze how the lack of electrical charge in neutrons affects their interaction with the nucleus. Neutrons can penetrate the nucleus without being repelled by the positive charge of the protons in the nucleus.
Step 5: Compare this with protons and alpha particles, which experience electrostatic repulsion from the positively charged nucleus, making it more difficult for them to initiate nuclear transmutations.
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Charge of Particles
Neutrons are electrically neutral particles, meaning they do not carry a charge. This lack of charge allows them to approach atomic nuclei without experiencing electrostatic repulsion, which is a significant barrier for charged particles like protons and alpha particles. In contrast, protons, which are positively charged, are repelled by the positively charged nucleus, making their interaction less favorable.
Recommended video:
Guided course
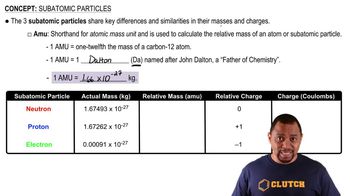
Subatomic Particles
Nuclear Forces
Nuclear forces are the strong interactions that hold protons and neutrons together within an atomic nucleus. Neutrons can effectively penetrate the nucleus due to their neutral charge, allowing them to be influenced by these strong nuclear forces without the hindrance of repulsion. This makes nuclear transmutations involving neutrons more feasible compared to those involving charged particles.
Recommended video:
Guided course
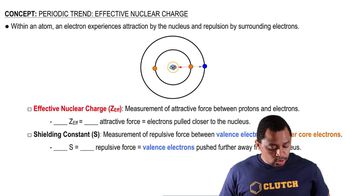
Effective Nuclear Charge
Distance and Interaction
The interaction between particles and the nucleus is influenced by distance. Neutrons can be attracted to the nucleus even from a distance due to the strong nuclear force, which operates over short ranges. In contrast, protons and alpha particles experience repulsion at greater distances due to their positive charge, making it more challenging for them to approach and interact with the nucleus effectively.
Recommended video:
Guided course
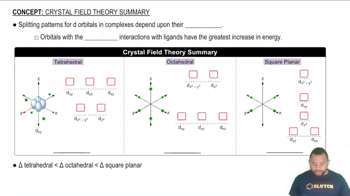
The greatest ligand-orbital interactions result in the greatest increase in energy.
Related Practice
Textbook Question
2
views
Textbook Question
Which of the following nuclides would you expect to be radioactive: 5826Fe, 6027Co, 9241Nb, mercury-202, radium-226? Justify your choices.
1
views
Textbook Question
Complete and balance the following nuclear equations by supplying the missing particle: (b) 21H + 32He → 42He + ?
1
views
Textbook Question
Complete and balance the following nuclear equations by supplying the missing particle: (d) 12253I → 12254Xe + ?
