(d) Why are active metals such as Al obtained by electrolysis using molten salts rather than aqueous solutions?
Ch.20 - Electrochemistry

Brown15th EditionChemistry: The Central ScienceISBN: 9780137542970Not the one you use?Change textbook
Chapter 20, Problem 96
Metallic magnesium can be made by the electrolysis of molten MgCl2. (a) What mass of Mg is formed by passing a current of 4.55 A through molten MgCl2 for 4.50 days? (b) How many minutes are needed to plate out 25.00 g Mg from molten MgCl2 using 3.50 A of current?
 Verified step by step guidance
Verified step by step guidance1
Step 1: Determine the total charge passed through the system using the formula Q = I \times t, where Q is the charge in coulombs, I is the current in amperes, and t is the time in seconds. Convert the given time from days to seconds for part (a) and from minutes to seconds for part (b).
Step 2: Use Faraday's laws of electrolysis to relate the charge to the amount of substance. The number of moles of electrons (n_e) is given by n_e = \frac{Q}{F}, where F is Faraday's constant (approximately 96485 C/mol).
Step 3: For the electrolysis of MgCl_2, determine the stoichiometry of the reaction. The half-reaction for the formation of magnesium is Mg^{2+} + 2e^- \rightarrow Mg. This indicates that 2 moles of electrons are required to produce 1 mole of Mg.
Step 4: Calculate the moles of Mg produced using the stoichiometry from Step 3. For part (a), use the moles of electrons calculated to find the moles of Mg. For part (b), use the given mass of Mg to find the moles of Mg using its molar mass.
Step 5: Convert the moles of Mg to mass for part (a) using the molar mass of Mg (24.305 g/mol). For part (b), use the moles of Mg and the stoichiometry to find the total charge required, then calculate the time in seconds and convert it to minutes.
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Electrolysis
Electrolysis is a chemical process that uses electrical energy to drive a non-spontaneous reaction. In the case of molten MgCl2, applying a current causes magnesium ions to migrate to the cathode, where they gain electrons and are reduced to form metallic magnesium. Understanding the principles of electrolysis is essential for calculating the mass of magnesium produced and the time required for the reaction.
Recommended video:
Guided course

The Electrolytic Cell
Faraday's Laws of Electrolysis
Faraday's Laws of Electrolysis quantify the relationship between the amount of substance produced at an electrode and the quantity of electric charge passed through the electrolyte. The first law states that the mass of a substance altered at an electrode is directly proportional to the total electric charge passed. This principle is crucial for determining the mass of magnesium formed and the time needed for electrolysis.
Recommended video:
Guided course
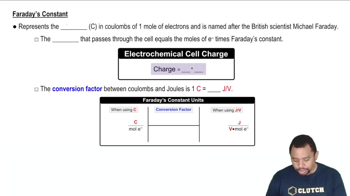
Faraday's Constant in Electrochemistry
Current and Time Relationship
The relationship between current, time, and charge is fundamental in electrolysis calculations. The total charge (Q) can be calculated using the formula Q = I × t, where I is the current in amperes and t is the time in seconds. This relationship allows for the conversion of current and time into the total charge, which is necessary for applying Faraday's laws to find the mass of magnesium produced or the time required for a specific mass.
Recommended video:
Guided course
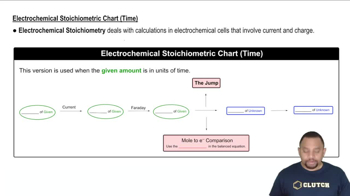
Electrochemical Stoichiometric Chart (Time)
Related Practice
Textbook Question
Textbook Question
(a) A Cr3+(aq) solution is electrolyzed, using a current of 7.60 A. What mass of Cr(s) is plated out after 2.00 days?
Textbook Question
(b) What amperage is required to plate out 0.250 mol Cr from a Cr3+ solution in a period of 8.00 h?
Textbook Question
(a) Calculate the mass of Li formed by electrolysis of molten LiCl by a current of 7.5 × 104 A flowing for a period of 24 h. Assume the electrolytic cell is 85% efficient. (b) What is the minimum voltage required to drive the reaction?
Textbook Question
A disproportionation reaction is an oxidation–reduction reaction in which the same substance is oxidized and reduced. Complete and balance the following disproportionation reactions:
(b) MnO42-(aq) → MnO4-(aq) + MnO2(s) (acidic solution)
