Textbook Question
(b) Do all voltaic cells produce a positive cell potential?
9
views

 Verified step by step guidance
Verified step by step guidance

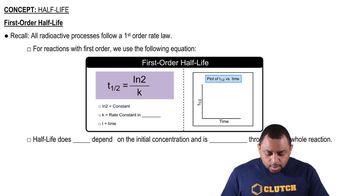

(b) Do all voltaic cells produce a positive cell potential?
(b) Write the half-reaction that occurs at a hydrogen electrode in acidic aqueous solution when it serves as the anode of a voltaic cell.
A voltaic cell that uses the reaction PdCl42-(aq) + Cd(s) → Pd(s) + 4 Cl-(aq) + Cd2+(aq) has a measured standard cell potential of +1.03 V. (a) Write the two half-cell reactions.
A voltaic cell that uses the reaction PdCl42-(aq) + Cd(s) → Pd(s) + 4 Cl-(aq) + Cd2+(aq) has a measured standard cell potential of +1.03 V. (b) By using data from Appendix E, determine E°red for the reaction involving Pd.