(b) Write the half-reaction that occurs at a hydrogen electrode in acidic aqueous solution when it serves as the anode of a voltaic cell.
Ch.20 - Electrochemistry

Brown15th EditionChemistry: The Central ScienceISBN: 9780137542970Not the one you use?Change textbook
Chapter 20, Problem 40a
A voltaic cell that uses the reaction PdCl42-(aq) + Cd(s) → Pd(s) + 4 Cl-(aq) + Cd2+(aq) has a measured standard cell potential of +1.03 V. (a) Write the two half-cell reactions.
 Verified step by step guidance
Verified step by step guidance1
Identify the oxidation and reduction components of the overall reaction. In this case, PdCl4^2- is reduced to Pd, and Cd is oxidized to Cd^2+.
Write the reduction half-reaction. Since PdCl4^2- is reduced to Pd, the palladium ion gains electrons. The equation will show the reduction of PdCl4^2- to Pd with the release of Cl^- ions.
Balance the reduction half-reaction for atoms other than hydrogen and oxygen. Ensure that the number of Pd and Cl atoms are equal on both sides of the equation.
Write the oxidation half-reaction. Since Cd is oxidized to Cd^2+, the cadmium metal loses electrons. The equation will show Cd transforming into Cd^2+.
Balance the oxidation half-reaction for atoms and charge. Ensure that the number of Cd atoms and the overall charge are equal on both sides of the equation.

Verified video answer for a similar problem:
This video solution was recommended by our tutors as helpful for the problem above.
Video duration:
4mWas this helpful?
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Half-Cell Reactions
Half-cell reactions represent the oxidation and reduction processes occurring in a voltaic cell. Each half-cell reaction shows the transfer of electrons, with one species being oxidized (losing electrons) and another being reduced (gaining electrons). In the given reaction, identifying the species that undergo oxidation and reduction is essential for writing the half-cell reactions.
Recommended video:
Guided course
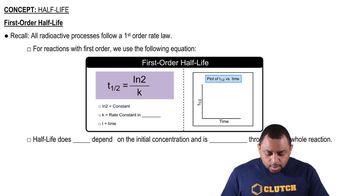
First-Order Half-Life
Standard Cell Potential
The standard cell potential (E°) is the measure of the voltage produced by a voltaic cell under standard conditions (1 M concentration, 1 atm pressure, and 25°C). It indicates the driving force behind the electrochemical reaction, with positive values suggesting a spontaneous reaction. Understanding how to interpret and calculate standard cell potentials is crucial for analyzing the overall cell reaction.
Recommended video:
Guided course

Standard Cell Potential
Electrochemical Series
The electrochemical series is a list of standard electrode potentials for various half-reactions, arranged from the most positive to the most negative. This series helps predict the direction of electron flow in a voltaic cell and the feasibility of reactions. By comparing the standard potentials of the half-reactions involved, one can determine which species will be oxidized and which will be reduced in the cell.
Recommended video:
Guided course

Electrochemical Cells
Related Practice
Textbook Question
Textbook Question
(c) Why is it impossible to measure the standard reduction potential of a single half-reaction?
Textbook Question
A voltaic cell that uses the reaction PdCl42-(aq) + Cd(s) → Pd(s) + 4 Cl-(aq) + Cd2+(aq) has a measured standard cell potential of +1.03 V. (b) By using data from Appendix E, determine E°red for the reaction involving Pd.
Textbook Question
A voltaic cell that uses the reaction PdCl42-(aq) + Cd(s) → Pd(s) + 4 Cl-(aq) + Cd2+(aq) has a measured standard cell potential of +1.03 V. (c) Sketch the voltaic cell, label the anode and cathode, and indicate the direction of electron flow
Textbook Question
Using standard reduction potentials (Appendix E), calculate the standard emf for each of the following reactions: (a) Cl21g2 + 2 I-1aq2 ¡ 2 Cl-1aq2 + I21s2
