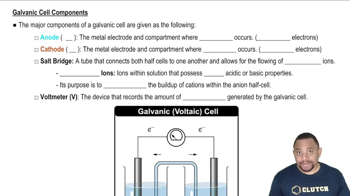
Textbook Question
A voltaic cell similar to that shown in Figure 20.5 is constructed. One electrode half-cell consists of a silver strip placed in a solution of AgNO3, and the other has an iron strip placed in a solution of FeCl2. The overall cell reaction is Fe1s2 + 2 Ag+1aq2 ¡ Fe2+1aq2 + 2 Ag1s2 (f) In which directions do the cations and anions migrate through the solution?