Textbook Question
What are the molecular and empirical formulas for each of the following compounds? Write the molecular formula for the following compound.

 Verified step by step guidance
Verified step by step guidance
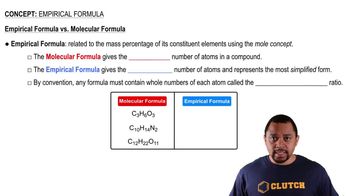
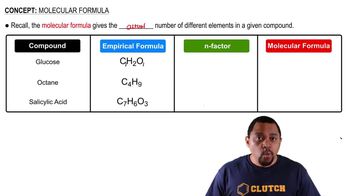
What are the molecular and empirical formulas for each of the following compounds? Write the molecular formula for the following compound.
Two substances have the same molecular and empirical formulas. Does this mean that they must be the same compound?
Write the empirical formula corresponding to each of the following molecular formulas: (a) Al2Br6
Write the empirical formula corresponding to each of the following molecular formulas: (c) C4H8O2
Write the empirical formula corresponding to each of the following molecular formulas: (d) P4O10 (e) C6H4Cl2 (f) B3N3H6.
Determine the molecular and empirical formulas of the following: (a) the organic solvent benzene, which has six carbon atoms and six hydrogen atoms