Each of the following elements is capable of forming an ion in chemical reactions. By referring to the periodic table, predict the charge of the most stable ion of each: c. K
Ch.2 - Atoms, Molecules, and Ions

Brown15th EditionChemistry: The Central ScienceISBN: 9780137542970Not the one you use?Change textbook
Chapter 2, Problem 59a,b
Using the periodic table to guide you, predict the chemical formula and name of the compound formed by the following elements: (a) Ga and F (b) Li and H
 Verified step by step guidance
Verified step by step guidance1
Step 1: Identify the type of bond that will form between the elements. Lithium (Li) is a metal and Hydrogen (H) is a non-metal. Therefore, they will form an ionic bond.
Step 2: Determine the charges of the ions. Lithium (Li) is in Group 1 of the periodic table and will lose one electron to form a +1 ion. Hydrogen (H) is also in Group 1 and will gain one electron to form a -1 ion.
Step 3: Balance the charges to form a neutral compound. In this case, the charges of the ions are already balanced (+1 and -1), so the formula will be LiH.
Step 4: Name the compound. The metal is named first, followed by the non-metal with an '-ide' ending. Therefore, the compound is named Lithium Hydride.

Verified video answer for a similar problem:
This video solution was recommended by our tutors as helpful for the problem above.
Video duration:
42sWas this helpful?
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Periodic Table
The periodic table organizes elements based on their atomic number, electron configuration, and recurring chemical properties. It provides essential information about each element, including its reactivity and typical bonding behavior, which is crucial for predicting the formulas of compounds formed between elements.
Recommended video:
Guided course
Periodic Table Classifications
Ionic Bonding
Ionic bonding occurs when one atom donates an electron to another, resulting in the formation of charged ions. In the case of lithium (Li) and hydrogen (H), lithium, a metal, can lose an electron to form a Li+ ion, while hydrogen can gain an electron to form H-. This transfer of electrons leads to the formation of the ionic compound lithium hydride (LiH).
Recommended video:
Guided course
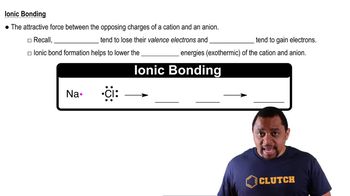
Chemical Bonds
Chemical Nomenclature
Chemical nomenclature is the system of naming chemical compounds based on their composition and structure. For ionic compounds like lithium hydride, the name is derived from the cation (Li+) followed by the anion (H-), resulting in the name 'lithium hydride.' Understanding nomenclature is essential for accurately communicating the identity of chemical substances.
Recommended video:
Guided course

Chemical Properties
Related Practice
Textbook Question
Textbook Question
Using the periodic table, predict the charge of the most stable ion of the following elements: d. Br
Textbook Question
Using the periodic table, predict the charge of the most stable ion of the following elements: e. Se
Textbook Question
Using the periodic table to guide you, predict the chemical formula and name of the compound formed by the following elements: (c) Al and I
Textbook Question
Using the periodic table to guide you, predict the chemical formula and name of the compound formed by the following elements: (d) K and S.
Textbook Question
The most common charge associated with scandium in its compounds is . Indicate the chemical formulas you would expect for compounds formed between scandium and b. sulfur
