Fill in the gaps in the following table:
Symbol 31P3-
Protons 34 50
Neutrons 45 69 118
Electrons 46 76
Net charge 2- 3+

 Verified step by step guidance
Verified step by step guidance
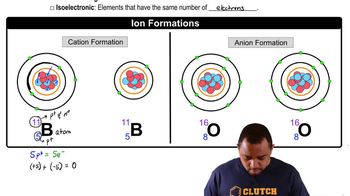

Fill in the gaps in the following table:
Symbol 31P3-
Protons 34 50
Neutrons 45 69 118
Electrons 46 76
Net charge 2- 3+
Each of the following elements is capable of forming an ion in chemical reactions. By referring to the periodic table, predict the charge of the most stable ion of each: c. K
Using the periodic table, predict the charge of the most stable ion of the following elements: d. Br
Using the periodic table to guide you, predict the chemical formula and name of the compound formed by the following elements: (a) Ga and F (b) Li and H
Using the periodic table to guide you, predict the chemical formula and name of the compound formed by the following elements: (c) Al and I
Using the periodic table to guide you, predict the chemical formula and name of the compound formed by the following elements: (d) K and S.