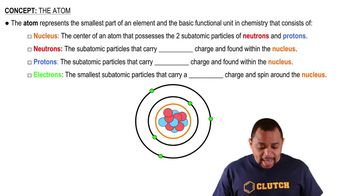
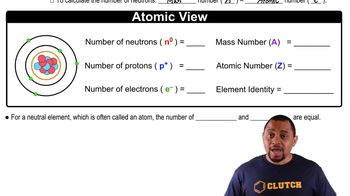
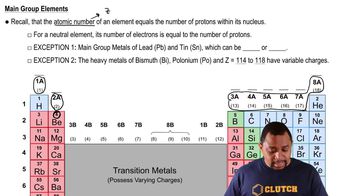
There are two different isotopes of bromine atoms. Under normal conditions, elemental bromine consists of molecules, and the mass of a molecule is the sum of the masses of the two atoms in the molecule. The mass spectrum of consists of three peaks: m/zRelative Peak Intensity157.836 0.2569 159.834 0.4999 161.832 0.2431
e. Calculate the abundances of the two isotopes.