The radius of an atom of gold (Au) is about 1.35 Å. c. If the atom is assumed to be a sphere, what is the volume in cm3 of a single Au atom?
Ch.2 - Atoms, Molecules, and Ions

Brown15th EditionChemistry: The Central ScienceISBN: 9780137542970Not the one you use?Change textbook
Chapter 2, Problem 21
Answer the following questions without referring to Table 2.1: (a) What are the main subatomic particles that make up the atom? (b) What is the relative charge (in multiples of the electronic charge) of each proton? (b) What is the relative charge (in multiples of the electronic charge) of each neutron? (b) What is the relative charge (in multiples of the electronic charge) of each electron? (c) Which of the particles is the most massive? (d) Which is the least massive?
 Verified step by step guidance
Verified step by step guidance1
Step 1: Identify the main subatomic particles that make up an atom, which are protons, neutrons, and electrons.
Step 2: Determine the relative charge of a proton. Protons have a positive charge, which is +1 in multiples of the electronic charge.
Step 3: Determine the relative charge of a neutron. Neutrons are neutral, meaning they have a charge of 0 in multiples of the electronic charge.
Step 4: Determine the relative charge of an electron. Electrons have a negative charge, which is -1 in multiples of the electronic charge.
Step 5: Compare the masses of the subatomic particles. Protons and neutrons have similar masses, with neutrons being slightly more massive, while electrons are much less massive than both protons and neutrons.
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Subatomic Particles
Atoms are composed of three main subatomic particles: protons, neutrons, and electrons. Protons are positively charged particles found in the nucleus, neutrons are neutral particles also located in the nucleus, and electrons are negatively charged particles that orbit the nucleus. Understanding these particles is fundamental to grasping atomic structure and behavior.
Recommended video:
Guided course
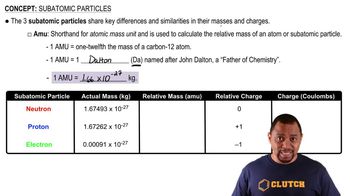
Subatomic Particles
Relative Charge of Subatomic Particles
The relative charges of subatomic particles are defined in multiples of the elementary charge (e). Protons carry a charge of +1e, neutrons have a charge of 0e, and electrons possess a charge of -1e. This charge distribution is crucial for understanding atomic interactions, bonding, and the overall stability of atoms.
Recommended video:
Guided course

Subatomic Particles
Mass of Subatomic Particles
In terms of mass, protons and neutrons are significantly more massive than electrons. A proton has a mass of approximately 1 atomic mass unit (amu), a neutron is slightly heavier at about 1 amu, while an electron has a mass of about 1/1836 amu, making it the least massive of the three. This mass difference plays a key role in the stability and structure of atoms.
Recommended video:
Guided course

Subatomic Particles
Related Practice
Textbook Question
Textbook Question
An atom of rhodium (Rh) has a diameter of about 2.7×10−8 cm. a. What is the radius of a rhodium atom in angstroms (Å) and in meters (m)?
Textbook Question
An atom of rhodium (Rh) has a diameter of about 2.7×10−8 cm. c. If you assume that the Rh atom is a sphere, what is the volume in m3 of a single atom?
Textbook Question
Determine whether each of the following statements is true or false. a. The nucleus has most of the mass and comprises most of the volume of an atom.
Textbook Question
Determine whether each of the following statements is true or false. b. Every atom of a given element has the same number of protons.
Textbook Question
Determine whether each of the following statements is true or false. c. The number of electrons in an atom equals the number of neutrons in the atom.
