Textbook Question
Determine whether each of the following statements is true or false. a. The nucleus has most of the mass and comprises most of the volume of an atom.

 Verified step by step guidance
Verified step by step guidance
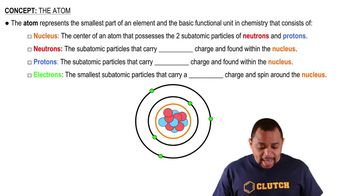
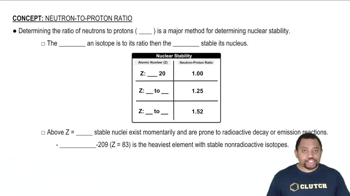

Determine whether each of the following statements is true or false. a. The nucleus has most of the mass and comprises most of the volume of an atom.
Determine whether each of the following statements is true or false. b. Every atom of a given element has the same number of protons.
Determine whether each of the following statements is true or false. d. The protons in the nucleus of the helium atom are held together by a force called the strong nuclear force.
Consider an atom of 10B. (a) How many protons, neutrons, and electrons does this atom contain?
Consider an atom of 10B. (b) What is the symbol of the atom obtained by adding one proton to 10B?