Consider the reaction PbCO3(s) ⇌ PbO(s) + CO2(g) Using data in Appendix C, calculate the equilibrium pressure of CO2 in the system at (a) 400 °C (b) 180 °C.

The value of Ka for nitrous acid (HNO2) at 25 °C is given in Appendix D. (c) What is the value of ΔG at equilibrium?
 Verified step by step guidance
Verified step by step guidance
Verified video answer for a similar problem:
Key Concepts
Acid Dissociation Constant (K<sub>a</sub>)

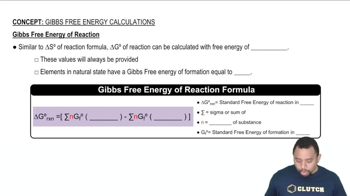
Gibbs Free Energy (ΔG)
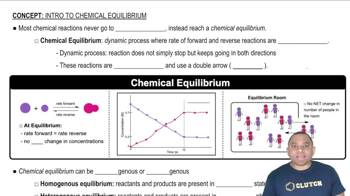
Equilibrium in Chemical Reactions
The value of Ka for nitrous acid (HNO2) at 25 °C is given in Appendix D. (a) Write the chemical equation for the equilibrium that corresponds to Ka.
The value of Ka for nitrous acid (HNO2) at 25 °C is given in Appendix D. (b) By using the value of Ka, calculate ΔG° for the dissociation of nitrous acid in aqueous solution.
The value of Ka for nitrous acid (HNO2) at 25 °C is given in Appendix D. (d) What is the value of ΔG when [H+] = 5.0⨉10-2 M, [NO2-] = 6.0⨉10-4 M, and [HNO2] = 0.20 M?
The Kb for methylamine (CH3NH2) at 25 °C is given in Appendix D. (a) Write the chemical equation for the equilibrium that corresponds to Kb.
The Kb for methylamine (CH3NH2) at 25 °C is given in Appendix D. (d) What is the value of ΔG when [H+] = 6.7 × 10-9 M, [CH3NH3+] = 2.4 × 10-3 M, and [CH3NH2] = 0.098 M?
