Indicate whether each statement is true or false. (a) Unlike enthalpy, where we can only ever know changes in H, we can know absolute values of S. (b) If you heat a gas such as CO2, you will increase its degrees of translational, rotational and vibrational motions. (c) CO2(g) and Ar(g) have nearly the same molar mass. At a given temperature, they will have the same number of microstates.
Ch.19 - Chemical Thermodynamics

Brown15th EditionChemistry: The Central ScienceISBN: 9780137542970Not the one you use?Change textbook
Chapter 19, Problem 41b
Predict the sign of the entropy change of the system for each of the following reactions: (b) CaCO3(s) → CaO(s) + CO2(g)
 Verified step by step guidance
Verified step by step guidance1
Identify the states of matter for each substance in the reaction: CaCO_3(s) is a solid, CaO(s) is a solid, and CO_2(g) is a gas.
Recognize that entropy, which is a measure of disorder or randomness, generally increases when a reaction produces more gas molecules.
Note that the reaction starts with one solid reactant and produces one solid and one gaseous product.
Understand that the production of a gas from a solid typically results in an increase in entropy because gases have higher entropy than solids.
Predict that the entropy change (ΔS) for the system is positive, as the formation of CO_2(g) increases the disorder of the system.

Verified video answer for a similar problem:
This video solution was recommended by our tutors as helpful for the problem above.
Video duration:
1mWas this helpful?
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Entropy
Entropy is a measure of the disorder or randomness in a system. In thermodynamics, it quantifies the number of ways a system can be arranged, with higher entropy indicating greater disorder. Understanding entropy is crucial for predicting the spontaneity of reactions and the direction in which they proceed.
Recommended video:
Guided course
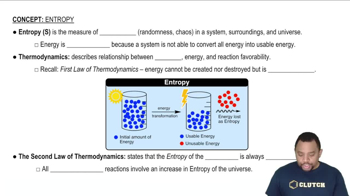
Entropy in Thermodynamics
Phase Changes and Gaseous Products
The phase of a substance significantly affects its entropy. Gases have much higher entropy than solids due to their greater freedom of movement and higher number of microstates. In the reaction given, the formation of a gas (CO2) from a solid (CaCO3) suggests an increase in entropy, as the system transitions from a more ordered state to a less ordered one.
Recommended video:
Guided course
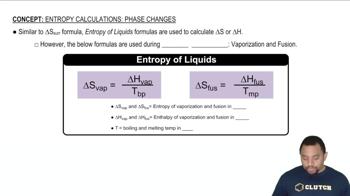
Entropy in Phase Changes
Thermodynamic Principles
Thermodynamic principles, particularly the second law, state that the total entropy of an isolated system can never decrease over time. This principle helps predict the sign of entropy changes in chemical reactions. In the reaction provided, the production of a gas from a solid indicates a positive change in entropy, aligning with the tendency for systems to evolve towards greater disorder.
Recommended video:
Guided course

First Law of Thermodynamics
Related Practice
Textbook Question
Textbook Question
Predict the sign of the entropy change of the system for each of the following reactions: (a) N2(g) + 3 H2(g) → 2 NH3(g)
Textbook Question
Predict the sign of the entropy change of the system for each of the following reactions: (c) 3 C2H2(g) → C6H6(g)
Textbook Question
Predict the sign of the entropy change of the system for each of the following reactions: (d) Al2O3(s) + 3 H2(g) → 2 Al(s) + 3 H2O(g)
Textbook Question
Predict the sign of ΔSsys for each of the following processes: (a) Molten gold solidifies.
