Predict the sign of the entropy change of the system for each of the following reactions: (b) CaCO3(s) → CaO(s) + CO2(g)
Ch.19 - Chemical Thermodynamics

Brown15th EditionChemistry: The Central ScienceISBN: 9780137542970Not the one you use?Change textbook
Chapter 19, Problem 42a
Predict the sign of ΔSsys for each of the following processes: (a) Molten gold solidifies.
 Verified step by step guidance
Verified step by step guidance1
Step 1: Understand the concept of entropy (ΔS). Entropy is a measure of the disorder or randomness in a system. A positive ΔS indicates an increase in disorder, while a negative ΔS indicates a decrease in disorder.
Step 2: Consider the process described: molten gold solidifying. This process involves a phase change from liquid to solid.
Step 3: Recall that in general, the transition from a liquid to a solid results in a decrease in entropy. This is because the particles in a solid are more ordered and have less freedom of movement compared to those in a liquid.
Step 4: Predict the sign of ΔSsys. Since the process involves a decrease in disorder (from liquid to solid), ΔSsys is expected to be negative.
Step 5: Conclude that the sign of ΔSsys for the solidification of molten gold is negative, indicating a decrease in entropy.

Verified video answer for a similar problem:
This video solution was recommended by our tutors as helpful for the problem above.
Video duration:
51sWas this helpful?
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Entropy (ΔS)
Entropy, denoted as ΔS, is a measure of the disorder or randomness in a system. In thermodynamics, a positive ΔS indicates an increase in disorder, while a negative ΔS signifies a decrease in disorder. Understanding how entropy changes during a process is crucial for predicting the spontaneity and direction of chemical reactions.
Recommended video:
Guided course
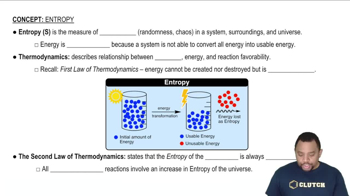
Entropy in Thermodynamics
Phase Changes
Phase changes refer to the transitions between solid, liquid, and gas states of matter. During these transitions, energy is absorbed or released, affecting the system's entropy. For example, when a substance solidifies, it typically moves from a more disordered liquid state to a more ordered solid state, which usually results in a decrease in entropy.
Recommended video:
Guided course
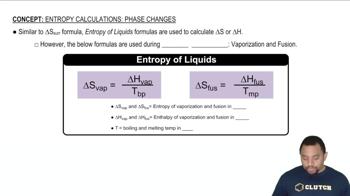
Entropy in Phase Changes
Spontaneity of Processes
The spontaneity of a process is determined by the changes in enthalpy (ΔH) and entropy (ΔS) according to the Gibbs free energy equation, ΔG = ΔH - TΔS. A process is spontaneous if ΔG is negative. In the case of molten gold solidifying, the decrease in entropy (negative ΔS) must be considered alongside the enthalpy change to assess the overall spontaneity of the process.
Recommended video:
Guided course
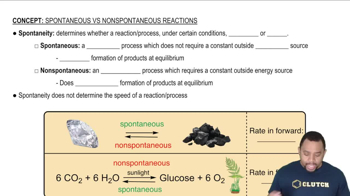
Spontaneity of Processes
Related Practice
Textbook Question
Textbook Question
Predict the sign of the entropy change of the system for each of the following reactions: (c) 3 C2H2(g) → C6H6(g)
Textbook Question
Predict the sign of the entropy change of the system for each of the following reactions: (d) Al2O3(s) + 3 H2(g) → 2 Al(s) + 3 H2O(g)
Textbook Question
Predict the sign of ΔSsys for each of the following processes: (b) Gaseous Cl2 dissociates in the stratosphere to form gaseous Cl atoms.
Textbook Question
Predict the sign of ΔSsys for each of the following processes: (c) Gaseous CO reacts with gaseous H2 to form liquid methanol, CH3OH.
Textbook Question
Predict the sign of ΔSsys for each of the following processes: (d) Calcium phosphate precipitates upon mixing Ca(NO3)2(aq) and (NH4)3PO4(aq).
