The fuel in high-efficiency natural-gas vehicles consists primarily of methane (CH4). (a) How much heat is produced in burning 1 mol of CH4(g) under standard conditions if reactants and products are brought to 298 K and H2O(l) is formed?
Ch.19 - Chemical Thermodynamics

Brown15th EditionChemistry: The Central ScienceISBN: 9780137542970Not the one you use?Change textbook
Chapter 19, Problem 76
Indicate whether ΔG increases, decreases, or does not change when the partial pressure of H₂ is increased in each of the following reactions: (a) N₂(g) + 3 H₂(g) ⇌ 2 NH₃(g) (b) 2 HBr(g) ⇌ H₂(g) + Br₂(g) (c) 2 H₂(g) + C₂H₂(g) ⇌ C₂H₆(g)
 Verified step by step guidance
Verified step by step guidance1
Step 1: Understand the relationship between Gibbs free energy (ΔG) and reaction quotient (Q). ΔG is related to the reaction quotient Q and the equilibrium constant K by the equation ΔG = ΔG° + RT ln(Q), where ΔG° is the standard Gibbs free energy change, R is the gas constant, and T is the temperature in Kelvin.
Step 2: Determine how the reaction quotient Q changes with an increase in the partial pressure of H₂. The reaction quotient Q is defined as the ratio of the products' partial pressures to the reactants' partial pressures, each raised to the power of their stoichiometric coefficients.
Step 3: Analyze reaction (a) N₂(g) + 3 H₂(g) ⇌ 2 NH₃(g). Increasing the partial pressure of H₂ will increase the denominator of Q, thus decreasing Q. Since Q < K, the reaction will shift towards the products, and ΔG will decrease.
Step 4: Analyze reaction (b) 2 HBr(g) ⇌ H₂(g) + Br₂(g). Increasing the partial pressure of H₂ will increase the numerator of Q, thus increasing Q. Since Q > K, the reaction will shift towards the reactants, and ΔG will increase.
Step 5: Analyze reaction (c) 2 H₂(g) + C₂H₂(g) ⇌ C₂H₆(g). Increasing the partial pressure of H₂ will increase the denominator of Q, thus decreasing Q. Since Q < K, the reaction will shift towards the products, and ΔG will decrease.
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Gibbs Free Energy (ΔG)
Gibbs Free Energy (ΔG) is a thermodynamic potential that measures the maximum reversible work obtainable from a thermodynamic system at constant temperature and pressure. It indicates the spontaneity of a reaction: a negative ΔG suggests a spontaneous process, while a positive ΔG indicates non-spontaneity. Understanding how ΔG changes with varying conditions, such as concentration or pressure, is crucial for predicting reaction behavior.
Recommended video:
Guided course
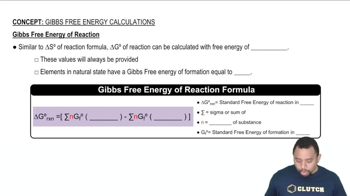
Gibbs Free Energy of Reactions
Le Chatelier's Principle
Le Chatelier's Principle states that if a dynamic equilibrium is disturbed by changing the conditions, the position of equilibrium shifts to counteract the change. In the context of gas reactions, increasing the partial pressure of a reactant will shift the equilibrium towards the products to reduce the effect of the added reactant, thereby influencing the ΔG of the reaction.
Recommended video:
Guided course

Le Chatelier's Principle
Reaction Quotient (Q) and Equilibrium Constant (K)
The reaction quotient (Q) is a measure of the relative concentrations of products and reactants at any point in a reaction, while the equilibrium constant (K) represents the ratio at equilibrium. Changes in partial pressures affect Q, and when Q is compared to K, it can indicate the direction in which the reaction will shift. This relationship is essential for determining how ΔG will change with variations in reactant or product concentrations.
Recommended video:
Guided course

Reaction Quotient Q
Related Practice
Textbook Question
Textbook Question
The fuel in high-efficiency natural-gas vehicles consists primarily of methane (CH4). (b) What is the maximum amount of useful work that can be accomplished under standard conditions by this system?
Textbook Question
Consider the reaction 2 NO2(g) → N2O4(g). (a) Using data from Appendix C, calculate ΔG° at 298 K. (b) Calculate ΔG at 298 K if the partial pressures of NO2 and N2O4 are 0.40 atm and 1.60 atm, respectively.
Textbook Question
Consider the reaction 3 CH4(g) → C3H8(g) + 2 H2(g). (a) Using data from Appendix C, calculate ΔG° at 298 K.
Textbook Question
Consider the reaction 3 CH4(g) → C3H8(g) + 2 H2(g). (b) Calculate ΔG at 298 K if the reaction mixture consists of 40.0 atm of CH4, 0.0100 atm of C3H8(g), and 0.0180 atm of H2.
