Textbook Question
Consider the reaction 2 NO2(g) → N2O4(g). (a) Using data from Appendix C, calculate ΔG° at 298 K. (b) Calculate ΔG at 298 K if the partial pressures of NO2 and N2O4 are 0.40 atm and 1.60 atm, respectively.

 Verified step by step guidance
Verified step by step guidance
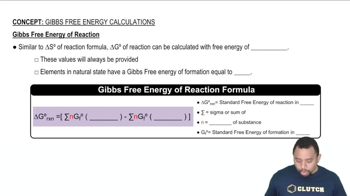


Consider the reaction 2 NO2(g) → N2O4(g). (a) Using data from Appendix C, calculate ΔG° at 298 K. (b) Calculate ΔG at 298 K if the partial pressures of NO2 and N2O4 are 0.40 atm and 1.60 atm, respectively.
Consider the reaction 3 CH4(g) → C3H8(g) + 2 H2(g). (a) Using data from Appendix C, calculate ΔG° at 298 K.
Using data from Appendix C, write the equilibrium-constant expression and calculate the value of the equilibrium constant and the free-energy change for these reactions at 298 K: (a) NaHCO3(s) ⇌ NaOH(s) + CO2(g)
Consider the decomposition of barium carbonate: BaCO3(s) ⇌ BaO(s) + CO2(g) Using data from Appendix C, calculate the equilibrium pressure of CO2 at (a) 298 K.