(a) Calculate the percent ionization of 0.125 M lactic acid (Ka = 1.4 × 10-4).

Use information from Appendix D to calculate the pH of: (a) a solution that is 0.250 M in sodium formate (HCOONa) and 0.100 M in formic acid (HCOOH); (b) a solution that is 0.510 M in pyridine (C5H5N) and 0.450 M in pyridinium chloride (C5H5NHCl); (c) a solution that is made by combining 55 mL of 0.050 M hydrofluoric acid with 125 mL of 0.10 M sodium fluoride.
 Verified step by step guidance
Verified step by step guidanceKey Concepts
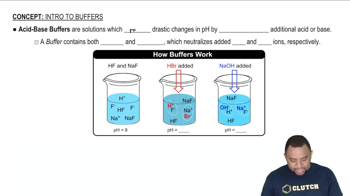
Buffer Solutions
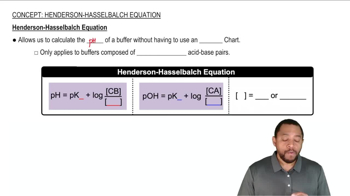
Henderson-Hasselbalch Equation
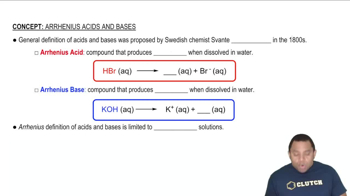
Acid-Base Equilibria
Which of these statements about the common-ion effect is most correct? (a) The solubility of a salt MA is decreased in a solution that already contains either M+ or A-. (b) Common ions alter the equilibrium constant for the reaction of an ionic solid with water. (c) The common-ion effect does not apply to unusual ions like SO32 - . (d) The solubility of a salt MA is affected equally by the addition of either A- or a noncommon ion.
Consider the equilibrium B(aq) + H2O(l) ⇌ HB+(aq) + OH–(aq). Suppose that a salt of HB+(aq) is added to a solution of B(aq) at equilibrium. (c) Will the pH of the solution increase, decrease, or stay the same?
a. Calculate the percent ionization of 0.007 M butanoic acid (𝐾𝑎=1.5×10−5).
(b) Calculate the percent ionization of 0.0075 M butanoic acid in a solution containing 0.085 M sodium butanoate.
