The value of Ksp for Cd(OH)2 is 2.5 × 10-14. (a) What is the molar solubility of Cd(OH)2?

A sample of 7.5 L of NH3 gas at 22 _x001F_C and 735 torr is bubbled into a 0.50-L solution of 0.40 M HCl. Assuming that all the NH3 dissolves and that the volume of the solution remains 0.50 L, calculate the pH of the resulting solution.
 Verified step by step guidance
Verified step by step guidanceKey Concepts
Ideal Gas Law

Acid-Base Neutralization
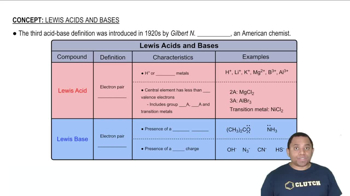
pH Calculation

The value of Ksp for Cd(OH)2 is 2.5 × 10–14. (b) The solubility of Cd(OH)2 can be increased through formation of the complex ion CdBr42- (Kf = 5 × 103). If solid Cd(OH)2 is added to a NaBr solution, what is the initial concentration of NaBr needed to increase the molar solubility of Cd(OH)2 to 1.0 × 10-3 mol/L?
(a) Write the net ionic equation for the reaction that occurs when a solution of hydrochloric acid (HCl) is mixed with a solution of sodium formate 1NaCHO22.
What is the pH at 25 C of water saturated with CO2 at a partial pressure of 1.10 atm? The Henry's law constant for CO2 at 25 C is 3.1 * 10-2 mol>L@atm.
The osmotic pressure of a saturated solution of strontium sulfate at 25 C is 21 torr. What is the solubility product of this salt at 25 C?
