Write an equation for the reaction in which H2C6H7O5-1aq2 acts as an acid in H2O1l2.

Write an equation for the reaction in which H2C6H7O5-1aq2 acts as a base in H2O1l2.
 Verified step by step guidance
Verified step by step guidance
Verified video answer for a similar problem:
Key Concepts
Acid-Base Theory

Dissociation of Water
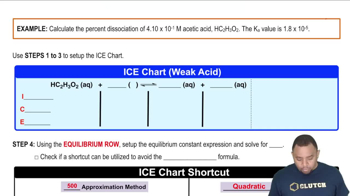
Chemical Equation Representation

Identify the Brønsted–Lowry acid and the Brønsted– Lowry base on the left side of each equation, and also identify the conjugate acid and conjugate base of each on the right side.
(a) HBrO(aq) + H2O(l) ⇌ H3O+(aq) + BrO-(aq)
(b) HSO4-(aq) + HCO3-(aq) ⇌ SO42-(aq) + H2CO3(aq)
(c) HSO3-(aq) + H3O+(aq) ⇌ H2SO3(aq) + H2O(l)
The hydrogen sulfite ion 1HSO3-2 is amphiprotic. Write a balanced chemical equation showing how it acts as an acid toward water and another equation showing how it acts as a base toward water.
What is the conjugate acid of HSO3-? What is its conjugate base?
Label each of the following as being a strong base, a weak base, or a species with negligible basicity. In each case write the formula of its conjugate acid, and indicate whether the conjugate acid is a strong acid, a weak acid, or a species with negligible acidity: (a) CH3COO-
Label each of the following as being a strong base, a weak base, or a species with negligible basicity. In each case write the formula of its conjugate acid, and indicate whether the conjugate acid is a strong acid, a weak acid, or a species with negligible acidity: (b) HCO3-
