Write an equation for the reaction in which H2C6H7O5-1aq2 acts as an acid in H2O1l2.

Label each of the following as being a strong base, a weak base, or a species with negligible basicity. In each case write the formula of its conjugate acid, and indicate whether the conjugate acid is a strong acid, a weak acid, or a species with negligible acidity: (b) HCO3-
 Verified step by step guidance
Verified step by step guidance
Verified video answer for a similar problem:
Key Concepts
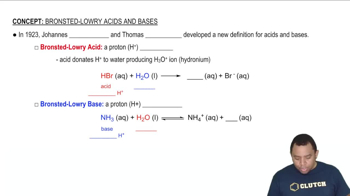
Acid-Base Theory
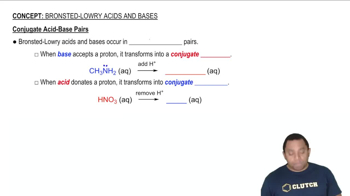
Conjugate Acid-Base Pairs
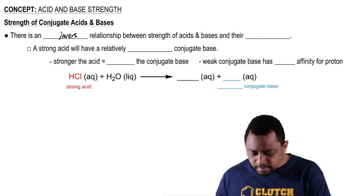
Strength of Acids and Bases
Write an equation for the reaction in which H2C6H7O5-1aq2 acts as a base in H2O1l2.
Label each of the following as being a strong base, a weak base, or a species with negligible basicity. In each case write the formula of its conjugate acid, and indicate whether the conjugate acid is a strong acid, a weak acid, or a species with negligible acidity: (a) CH3COO-
Label each of the following as being a strong base, a weak base, or a species with negligible basicity. In each case write the formula of its conjugate acid, and indicate whether the conjugate acid is a strong acid, a weak acid, or a species with negligible acidity: (c) O2-
Label each of the following as being a strong base, a weak base, or a species with negligible basicity. In each case write the formula of its conjugate acid, and indicate whether the conjugate acid is a strong acid, a weak acid, or a species with negligible acidity: (d) Cl-
Label each of the following as being a strong base, a weak base, or a species with negligible basicity. In each case write the formula of its conjugate acid, and indicate whether the conjugate acid is a strong acid, a weak acid, or a species with negligible acidity: (e) NH3.
