Consider the molecular models shown here, where X represents a halogen atom. (a) If X is the same atom in both molecules, which molecule will be more acidic?

Which of the following statements is false? (a) An Arrhenius base increases the concentration of OH- in water. (b) A Brønsted-Lowry base is a proton acceptor. (c) Water can act as a Brønsted–Lowry acid. (d) Water can act as a Brønsted–Lowry base. (e) Any compound that contains an –OH group acts as a Brønsted-Lowry base.
 Verified step by step guidance
Verified step by step guidance
Verified video answer for a similar problem:
Key Concepts
Arrhenius Theory of Acids and Bases
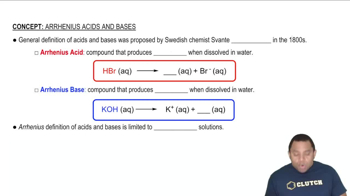
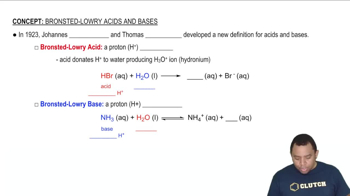
Brønsted-Lowry Theory of Acids and Bases
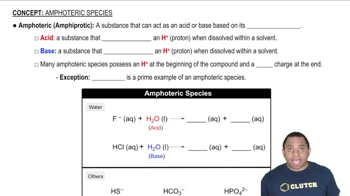
Amphoteric Substances
Consider the molecular models shown here, where X represents a halogen atom. (b) Does the acidity of each molecule increase or decrease as the electronegativity of the atom X increases?
NH31g2 and HCl(g) react to form the ionic solid NH4Cl1s2. Which substance is the Brønsted–Lowry acid in this reaction? Which is the Brønsted–Lowry base?
Identify the Lewis acid and Lewis base among the reactants in each of the following reactions:
(a) Fe(ClO4)3(s) + 6 H2O(l) ⇌ [Fe(H2O)6]3+(aq) + 3 ClO4-(aq)
(b) CN-(aq) + H2O(l) ⇌ HCN(aq) + OH-(aq)
(c) (CH3)3N(g) + BF3(g) ⇌ (CH3)NBF3(s)
(d) HIO(lq) + NH2-(lq) ⇌ NH3(lq) + IO-(lq) (lq denotes liquid ammonia as solvent)
Identify the Lewis acid and Lewis base in each of the following reactions:
(a) HNO2(aq) + OH-(aq) ⇌ NO2-(aq) + H2O(l)
(b) FeBr3(s) + Br-(aq) ⇌ FeBr4-(aq)
(c) Zn2+(aq) + 4 NH3(aq) ⇌ Zn(NH3)42+(aq)
(d) SO2(g) + H2O(l) ⇌ H2SO3(aq)
Give the conjugate base of the following Brønsted–Lowry acids: (i) HIO3, (ii) NH4+.
