Give the conjugate base of the following Brønsted–Lowry acids: (i) HIO3, (ii) NH4+.
Ch.16 - Acid-Base Equilibria

Brown15th EditionChemistry: The Central ScienceISBN: 9780137542970Not the one you use?Change textbook
Chapter 16, Problem 21
Identify the Brønsted–Lowry acid and the Brønsted–Lowry base on the left side of each of the following equations, and also identify the conjugate acid and conjugate base of each on the right side:
(a) NH4+(aq) + CN-(aq) ⇌ HCN(aq) + NH3(aq)
(b) (CH3)3N(aq) + H2O(l) ⇌ (CH3)3NH+(aq) + OH-(aq)
(c) HCOOH(aq) + PO43-(aq) ⇌ HCOO-(aq)+ HPO42-(aq)
 Verified step by step guidance
Verified step by step guidance1
Identify the species that donates a proton (H⁺) on the left side of the equation. This species is the Brønsted–Lowry acid.
Identify the species that accepts a proton (H⁺) on the left side of the equation. This species is the Brønsted–Lowry base.
On the right side of the equation, identify the species that results from the Brønsted–Lowry base gaining a proton. This is the conjugate acid.
On the right side of the equation, identify the species that results from the Brønsted–Lowry acid losing a proton. This is the conjugate base.
Verify that the transfer of a proton from the acid to the base is consistent with the formation of the conjugate acid and conjugate base.

Verified video answer for a similar problem:
This video solution was recommended by our tutors as helpful for the problem above.
Video duration:
2mWas this helpful?
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Brønsted–Lowry Acid-Base Theory
The Brønsted–Lowry theory defines acids as proton donors and bases as proton acceptors. This framework allows for the identification of acid-base reactions based on the transfer of protons (H+ ions) between species. In a reaction, the substance that donates a proton is the acid, while the one that accepts it is the base.
Recommended video:
Guided course

Bronsted-Lowry Acid-Base Theory
Conjugate Acid-Base Pairs
A conjugate acid-base pair consists of two species that differ by the presence of a proton. When a Brønsted–Lowry acid donates a proton, it forms its conjugate base, and when a base accepts a proton, it forms its conjugate acid. Understanding these pairs is crucial for analyzing acid-base reactions and predicting the direction of equilibrium.
Recommended video:
Guided course
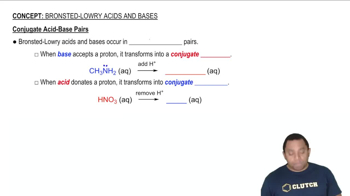
Conjugate Acid-Base Pairs
Chemical Equations and Reaction Dynamics
Chemical equations represent the reactants and products in a reaction, showing how substances interact. In the context of acid-base reactions, it is important to identify the roles of each species on both sides of the equation. This includes recognizing which species are acids, bases, conjugate acids, and conjugate bases, which helps in understanding the overall reaction dynamics.
Recommended video:
Guided course

Balancing Chemical Equations
Related Practice
Textbook Question
Textbook Question
Give the conjugate acid of the following Brønsted–Lowry bases: (i) SO42-, (ii) CH3NH2.
Textbook Question
Give the conjugate base of the following Brønsted–Lowry acids: (i) HCOOH, (ii) HPO42-.
Textbook Question
Identify the Brønsted–Lowry acid and the Brønsted– Lowry base on the left side of each equation, and also identify the conjugate acid and conjugate base of each on the right side.
(a) HBrO(aq) + H2O(l) ⇌ H3O+(aq) + BrO-(aq)
(b) HSO4-(aq) + HCO3-(aq) ⇌ SO42-(aq) + H2CO3(aq)
(c) HSO3-(aq) + H3O+(aq) ⇌ H2SO3(aq) + H2O(l)
Textbook Question
The hydrogen sulfite ion 1HSO3-2 is amphiprotic. Write a balanced chemical equation showing how it acts as an acid toward water and another equation showing how it acts as a base toward water.
Textbook Question
What is the conjugate acid of HSO3-? What is its conjugate base?
1
views
