Textbook Question
Calculate 3OH-4 for each of the following solutions, and indicate whether the solution is acidic, basic, or neutral: (a) 3H+4 = 0.0505 M

 Verified step by step guidance
Verified step by step guidance

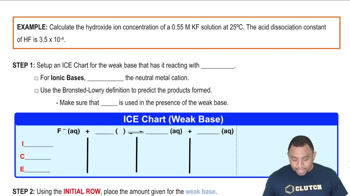
Calculate 3OH-4 for each of the following solutions, and indicate whether the solution is acidic, basic, or neutral: (a) 3H+4 = 0.0505 M
Calculate 3OH-4 for each of the following solutions, and indicate whether the solution is acidic, basic, or neutral: (b) 3H+4 = 2.5 * 10-10 M
Calculate 3OH-4 for each of the following solutions, and indicate whether the solution is acidic, basic, or neutral: (c) a solution in which 3H+4 is 1000 times greater than 3OH-4.
By what factor does [H+] change for a pH change of (a) 2.00 units? (b) 0.50 units?