Addition of phenolphthalein to an unknown colorless solution does not cause a color change. The addition of bromthymol blue to the same solution leads to a yellow color. (b) Which of the following can you establish about the solution: (i) A minimum pH, (ii) A maximum pH, or (iii) A specific range of pH values?
Ch.16 - Acid-Base Equilibria

Brown15th EditionChemistry: The Central ScienceISBN: 9780137542970Not the one you use?Change textbook
Chapter 16, Problem 46
Determine whether each of the following is true or false: (a) All strong bases are salts of the hydroxide ion. (b) The addition of a strong base to water produces a solution of pH > 7.0. (c) Because Mg(OH)2 is not very soluble, it cannot be a strong base.
 Verified step by step guidance
Verified step by step guidance1
Step 1: Understand the definition of a strong base. A strong base is a compound that completely dissociates into its ions in water. Common strong bases include hydroxides of alkali metals and some alkaline earth metals.
Step 2: Analyze statement (a). Strong bases are typically metal hydroxides that dissociate completely in water, forming hydroxide ions (OH^-). Consider whether all strong bases fit this description.
Step 3: Evaluate statement (b). Recall that the pH scale measures the acidity or basicity of a solution, with pH > 7 indicating a basic solution. Consider the effect of adding a strong base to water on the pH.
Step 4: Examine statement (c). Consider the solubility of Mg(OH)_2 and its dissociation in water. A strong base dissociates completely, but solubility affects the concentration of ions in solution.
Step 5: Summarize your findings for each statement, determining whether each is true or false based on the definitions and properties of strong bases.
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Strong Bases
Strong bases are substances that completely dissociate in water to produce hydroxide ions (OH-). Common examples include sodium hydroxide (NaOH) and potassium hydroxide (KOH). While many strong bases are hydroxides, not all strong bases are exclusively defined as salts of the hydroxide ion, as some can be derived from other compounds.
Recommended video:
Guided course

Strong Acid-Strong Base Titration
pH Scale
The pH scale measures the acidity or basicity of a solution, with values ranging from 0 to 14. A pH of 7 is neutral, while values above 7 indicate basic solutions. The addition of a strong base to water typically results in a solution with a pH greater than 7, reflecting the increased concentration of hydroxide ions.
Recommended video:
Guided course
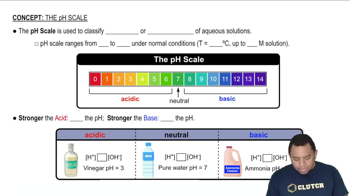
The pH Scale
Solubility and Strong Bases
Solubility refers to the ability of a substance to dissolve in a solvent, such as water. While strong bases are characterized by their complete dissociation in solution, some compounds, like magnesium hydroxide (Mg(OH)2), are not very soluble. This does not disqualify them from being classified as strong bases; rather, it indicates that their strength is not solely dependent on solubility.
Recommended video:
Guided course

Strong Acid-Strong Base Titration
Related Practice
Textbook Question
Textbook Question
Addition of phenolphthalein to an unknown colorless solution does not cause a color change. The addition of bromthymol blue to the same solution leads to a yellow color. (c) What other indicator or indicators would you want to use to determine the pH of the solution more precisely?
Textbook Question
Calculate the pH of each of the following strong acid solutions: (b) 1.52 g of HNO3 in 575 mL of solution
Textbook Question
Calculate the pH of each of the following strong acid solutions: (b) 0.225 g of HClO3 in 2.00 L of solution
